Page 1580 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1580
Chapter 86 Plasma Cell Neoplasms 1407
100 1.00
P = .03 by Wilcoxon test
P = .04 by log-rank test
Overall survival (%) 50 Conventional Survival 0.50 Intensive therapy
High-dose
0.75
75
dose
25
7 yr SDT HD P 0.25 Standard therapy
OS 25% 43% .03
0 0.00
0 15 30 45 60 0 20 40 60 80
A Months B Months
Proportion alive 30 mo 45 mo 60 mo No. at risk
Conventional (%) 63 (53-73) 35 (22-50) 12 (1-40) High-dose 201 148 79 38 8
High-dose (%) 69 (58-78) 61 (50-71) 52 (36-67) Standard 200 129 70 30 8
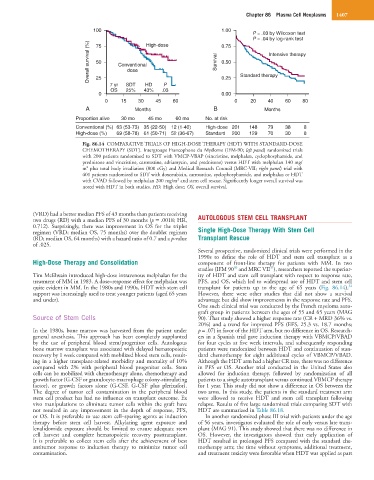
Fig. 86.14 COMPARATIVE TRIALS OF HIGH-DOSE THERAPY (HDT) WITH STANDARD-DOSE
CHEMOTHERAPY (SDT). Intergroupe Francophone du Myélome (IFM-90; left panel) randomized trials
with 200 patients randomized to SDT with VMCP-VBAP (vincristine, melphalan, cyclophosphamide, and
prednisone and vincristine, carmustine, adriamycin, and prednisone) versus HDT with melphalan 140 mg/
2
m plus total body irradiation (800 cGy) and Medical Research Council (MRC-VII; right panel) trial with
401 patients randomized to SDT with doxorubicin, carmustine, cyclophosphamide, and melphalan or HDT
2
with CVAD followed by melphalan 200 mg/m and stem cell rescue. Significantly longer overall survival was
noted with HDT in both studies. HD, High dose; OS, overall survival.
(VRD) had a better median PFS of 43 months than patients receiving
two drugs (RD) with a median PFS of 30 months (p = .0018; HR, AUTOLOGOUS STEM CELL TRANSPLANT
0.712). Surprisingly, there was improvement in OS for the triplet
regimen (VRD; median OS, 75 months) over the doublet regimen Single High-Dose Therapy With Stem Cell
(RD; median OS, 64 months) with a hazard ratio of 0.7 and a p-value Transplant Rescue
of .025.
Several prospective, randomized clinical trials were performed in the
1990s to define the role of HDT and stem cell transplant as a
High-Dose Therapy and Consolidation component of front-line therapy for patients with MM. In two
30
31
studies (IFM 90 and MRC VII ), researchers reported the superior-
Tim McElwain introduced high-dose intravenous melphalan for the ity of HDT and stem cell transplant with respect to response rate,
treatment of MM in 1983. A dose–response effect for melphalan was PFS, and OS, which led to widespread use of HDT and stem cell
32
quite evident in MM. In the 1980s and 1990s, HDT with stem cell transplant for patients up to the age of 65 years (Fig. 86.14).
support was increasingly used to treat younger patients (aged 65 years However, there were other studies that did not show a survival
and under). advantage but did show improvements in the response rate and PFS.
One such clinical trial was conducted by the French myeloma auto-
graft group in patients between the ages of 55 and 65 years (MAG
Source of Stem Cells 90). That study showed a higher response rate (CR + MRD 36% vs.
20%) and a trend for improved PFS (EFS, 25.3 vs. 18.7 months;
In the 1980s, bone marrow was harvested from the patient under p = .07) in favor of the HDT arm, but no difference in OS. Research-
general anesthesia. This approach has been completely supplanted ers in a Spanish trial gave induction therapy with VBMCP/VBAD
by the use of peripheral blood stem/progenitor cells. Autologous for four cycles at five week intervals, and subsequently responding
bone marrow transplant was associated with delayed hematopoietic patients were randomized between HDT and continuation of stan-
recovery by 1 week compared with mobilized blood stem cells, result- dard chemotherapy for eight additional cycles of VBMCP/VBAD.
ing in a higher transplant-related morbidity and mortality of 10% Although the HDT arm had a higher CR rate, there was no difference
compared with 2% with peripheral blood progenitor cells. Stem in PFS or OS. Another trial conducted in the United States also
cells can be mobilized with chemotherapy alone, chemotherapy and allowed for induction therapy, followed by randomization of all
growth factor (G-CSF or granulocyte-macrophage colony-stimulating patients to a single autotransplant versus continued VBMCP therapy
factor), or growth factors alone (G-CSF, G-CSF plus plerixafor). for 1 year. This study did not show a difference in OS between the
The degree of tumor cell contamination in the peripheral blood two arms. In this study, the patients in the standard treatment arm
stem cell product has had no influence on transplant outcome. Ex were allowed to receive HDT and stem cell transplant following
vivo manipulations to eliminate tumor cells within the graft have relapse. Results of five large randomized trials comparing SDT with
not resulted in any improvement in the depth of response, PFS, HDT are summarized in Table 86.18.
or OS. It is preferable to use stem cell–sparing agents as induction In another randomized phase III trial with patients under the age
therapy before stem cell harvest. Alkylating agent exposure and of 56 years, investigatos evaluated the role of early versus late trans-
lenalidomide exposure should be limited to ensure adequate stem plant (MAG 91). This study showed that there was no difference in
cell harvest and complete hematopoietic recovery posttransplant. OS. However, the investigators showed that early application of
It is preferable to collect stem cells after the achievement of best HDT resulted in prolonged PFS compared with the standard che-
antitumor response to induction therapy to minimize tumor cell motherapy arm; the time without symptoms, additional treatment,
contamination. and treatment toxicity were favorable when HDT was applied as part