Page 2102 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2102
Chapter 124 Megakaryocyte and Platelet Structure 1865
PLATELETS localized in the OCS of the resting platelet, awaiting movement
to the surface when the cells are activated. Although contiguous with
Structure of the Resting Platelet the plasma membrane, not all proteins on the cell surface can enter
the OCS. Factors controlling movement into the OCS remain to be
Megakaryocyte development culminates in the release of mature defined but likely depend on the actin cytoskeleton. Entry restriction,
discoid platelets having dimensions of approximately 3.0 × 0.5 µm however, occurs at the necks of OCS infoldings. The third function
23
and a cytoplasmic volume of 7 fL. The evolutionary explanation for of the OCS is to serve as a source of redundant plasma membrane
the discoid shape of the platelet is unknown. Discoid shape may for cell spreading. OCS membrane initially is disgorged to the surface
permit more efficient flow or dispersion of clot-promoting elements following cell activation. When cells are activated in solution, much
or may simply reflect the microtubule-based mechanism by which of this membrane is subsequently reabsorbed into the remnants of
platelets are produced. In humans, platelets, once released from the the OCS.
ends of proplatelets, normally circulate for 7–10 days. Given that A small thin zone of cytoplasm separates the plasma membrane
nearly 1 trillion platelets circulate in an adult human, each day an of the resting platelet from a marginal microtubule coil and the
adult produces approximately 100 billion platelets. general intracellular space, which contains all inclusion bodies and
The precise morphology of newly released platelets is unknown. the internal cytoskeleton of the cell. This zone is filled with the
However, when released into the circulation or maintained in culture, spectrin-based membrane skeleton (see Fig. 124.9A). Beneath this
platelets have a very reproducible structure. Although they are het- zone sits a microtubule coil. Then follows the cytoplasmic space,
erogeneous in size, presumably because of changes in size as they age, which is filled with filaments of actin that embed granules, organelles,
platelets have discoid shapes with flat, featureless surfaces (Fig. the OCS, and other specialized membrane systems such as smooth
124.8A and Fig. 124.9A) that are interrupted only by pit-like open- endoplasmic reticulum.
ings into the open canalicular system (OCS). The OCS is an extensive Platelets actively recruit other blood-borne cells to areas of
system of internal membrane conduits that serves as a passageway to vascular damage by releasing mediators packaged in intracellular
the outside world into which granular contents are released. It also is granules (described earlier in Cytoplasmic Maturation) that initiate
a reservoir of plasma membrane, membrane receptors, and proteins. secondary homeostatic interactions and that express a “sticky” apical
For example, approximately 30% of the thrombin receptors are surface after the platelets adhere. In the resting platelet, granules
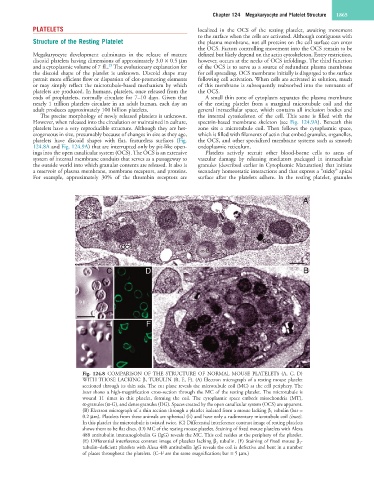
Fig. 124.8 COMPARISON OF THE STRUCTURE OF NORMAL MOUSE PLATELETS (A, C, D)
WITH THOSE LACKING β 1 TUBULIN (B, E, F). (A) Electron micrograph of a resting mouse platelet
sectioned through its thin axis. The cut plane reveals the microtubule coil (MC) at the cell periphery. The
inset shows a high-magnification cross-section through the MC of the resting platelet. The microtubule is
wound 11 times in this platelet, forming the coil. The cytoplasmic space embeds mitochondria (MT),
α-granules (α-G), and dense granules (DG). Spaces created by the open canalicular system (OCS) are apparent.
(B) Electron micrograph of a thin section through a platelet isolated from a mouse lacking β 1 tubulin (bar =
0.2 µm). Platelets from these animals are spherical (E) and have only a rudimentary microtubule coil (inset).
In this platelet the microtubule is twisted twice. (C) Differential interference contrast image of resting platelets
shows them to be flat discs. (D) MC of the resting mouse platelet. Staining of fixed mouse platelets with Alexa
488 antitubulin immunoglobulin G (IgG) reveals the MC. This coil resides at the periphery of the platelet.
(E) Differential interference contrast image of platelets lacking β 1 tubulin. (F) Staining of fixed mouse β 1-
tubulin–deficient platelets with Alexa 488 antitubulin IgG reveals the coil is defective and bent in a number
of places throughout the platelets. (C–F are the same magnification; bar = 5 µm.)