Page 1763 - Williams Hematology ( PDFDrive )
P. 1763
1738 Part XI: Malignant Lymphoid Diseases Chapter 107: Myeloma 1739
MDSCs Myeloma cell GSK3b
FKHR
Akt mTOR
PI3K
Dendritic cells OPG PKC Migration
RANKL JAK/
STAT3 BCL-X L
MCL1
RANK
Raf MEK/ERK
MYC
BCL-X L
IAP
Osteoblasts IL-6
Osteoclasts Cyclin D1
CD138 Survival
Antiapoptosis
Proliferation
Migration
ECM
VLA4
VEGF
B-Lymphocytes IL-6 HGF
IGF-1
VCAM1
BMSCs VEGF
T-Lymphocytes HGF Angiogenesis
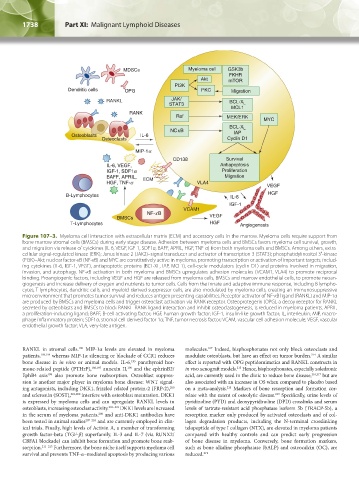
Figure 107–3. Myeloma cell interaction with extracellular matrix (ECM) and accessory cells in the marrow. Myeloma cells require support from
bone marrow stromal cells (BMSCs) during early stage disease. Adhesion between myeloma cells and BMSCs favors myeloma cell survival, growth,
and migration via release of cytokines (IL-6, VEGF, IGF-1, SDF1α, BAFF, APRIL, HGF, TNF-α) from both myeloma cells and BMSCs. Among others, extra-
cellular signal-regulated kinase (ERK); Janus kinase 2 (JAK2)–signal transducer and activator of transcription 3 (STAT3); phosphatidylinositol 3′-kinase
(PI3K)–Akt; nuclear factor-κB (NF-κB) and MYC are constitutively active in myeloma, promoting transcription or activation of important targets, includ-
ing cytokines (IL-6, IGF-1, VEGF), antiapoptotic proteins (BCL-XL, IAP, MCL1), cell-cycle modulators (cyclin D1) and proteins involved in migration,
invasion, and autophagy. NF-κB activation in both myeloma and BMSCs upregulates adhesion molecules (VCAM1, VLA4) to promote reciprocal
binding. Proangiogenic factors, including VEGF and HGF are released from myeloma cells, BMSCs and marrow endothelial cells, to promote neoan-
giogenesis and increase delivery of oxygen and nutrients to tumor cells. Cells from the innate and adaptive immune response, including B lympho-
cytes, T lymphocytes, dendritic cells, and myeloid-derived suppressor cells, are also modulated by myeloma cells, creating an immunosuppressive
microenvironment that promotes tumor survival and reduces antigen presenting capabilities. Receptor activator of NF-κB ligand (RANKL) and MIP-1α
are produced by BMSCs and myeloma cells and trigger osteoclast activation via RANK receptor. Osteoprotegerin (OPG), a decoy receptor for RANKL
secreted by osteoblasts and BMSCs to block RANKL–RANK ligand interaction and inhibit osteoclastogenesis, is reduced in myeloma patients. APRIL,
a proliferation-inducing ligand; BAFF, B-cell activating factor; HGF, human growth factor; IGF-1, insulin-like growth factor; IL, interleukin; MIP, macro-
phage inflammatory protein; SDF1α, stromal cell derived factor 1α; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular
endothelial growth factor; VLA, very-late antigen.
RANKL in stromal cells. MIP-1α levels are elevated in myeloma molecules. Indeed, bisphosphonates not only block osteoclasts and
190
167
patients, 193,194 whereas MIP-1α silencing or blockade of CCR1 reduces modulate osteoblasts, but have an effect on tumor burden. A similar
214
bone disease in in vitro or animal models. IL-6, parathyroid hor- effect is reported with OPG peptidomimetics and RANKL constructs in
195
mone-related peptide (PTHrP), 196,197 annexin II, and the ephrinB2/ in vivo xenograft models. Hence, bisphosphonates, especially zoledronic
198
215
EphB4 axis also promote bone reabsorption. Osteoblast suppres- acid, are currently used in the clinic to reduce bone disease, 216,217 but are
199
sion is another major player in myeloma bone disease: WNT signal- also associated with an increase in OS when compared to placebo based
ing antagonists, including DKK1, frizzled related protein-2 (FRP-2), on a meta-analysis. Markers of bone resorption and formation cor-
200
218
and sclerostin (SOST), 201,202 interfere with osteoblast maturation. DKK1 relate with the extent of osteolytic disease. Specifically, urine levels of
219
is expressed by myeloma cells and can upregulate RANKL levels in pyridinoline (PYD) and deoxypyridinoline (DPD) crosslinks and serum
osteoblasts, increasing osteoclast activity. 203–205 DKK1 levels are increased levels of tartrate-resistant acid phosphatase isoform 5b (TRACP-5b), a
in the serum of myeloma patients, and anti-DKK1 antibodies have resorption marker only produced by activated osteoclasts and of col-
206
been tested in animal studies 207–210 and are currently employed in clin- lagen degradation products, including the N-terminal crosslinking
ical trials. Finally, high levels of Activin A, a member of transforming telopeptide of type I collagen (NTX), are elevated in myeloma patients
growth factor-beta (TGF-β) superfamily, IL-3 and IL-7 (via RUNX2/ compared with healthy controls and can predict early progression
CBFA1 blockade) can inhibit bone formation and promote bone reab- of bone disease in myeloma. Conversely, bone formation markers,
sorption. 211–213 Furthermore, the bone niche itself supports myeloma cell such as bone alkaline phosphatase (bALP) and osteocalcin (OC), are
survival and prevents TNF-α–mediated apoptosis by producing various reduced. 219
Kaushansky_chapter 107_p1733-1772.indd 1738 9/21/15 12:34 PM