Page 257 - Williams Hematology ( PDFDrive )
P. 257
232 Part IV: Molecular and Cellular Hematology Chapter 16: Cell-Cycle Regulation and Hematologic Disorders 233
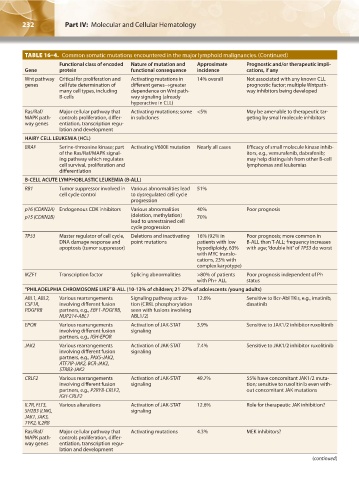
TABLE 16–4. Common somatic mutations encountered in the major lymphoid malignancies.(Continued)
Functional class of encoded Nature of mutation and Approximate Prognostic and/or therapeutic impli-
Gene protein functional consequence incidence cations, if any
Wnt pathway Critical for proliferation and Activating mutations in 14% overall Not associated with any known CLL
genes cell fate determination of different genes→greater prognostic factor; multiple Wntpath-
many cell types, including dependence on Wnt path- way inhibitors being developed
B-cells way signaling (already
hyperactive in CLL)
Ras/Raf/ Major cellular pathway that Activating mutations; some <5% May be amenable to therapeutic tar-
MAPK path- controls proliferation, differ- in subclones geting by small molecule inhibitors
way genes entiation, transcription regu-
lation and development
HAIRY CELL LEUKEMIA (HCL)
BRAF Serine-threonine kinase; part Activating V600E mutation Nearly all cases Efficacy of small molecule kinase inhib-
of the Ras/Raf/MAPK signal- itors, e.g., vemurafenib, dabrafenib;
ing pathway which regulates may help distinguish from other B-cell
cell survival, proliferation and lymphomas and leukemias
differentiation
B-CELL ACUTE LYMPHOBLASTIC LEUKEMIA (B-ALL)
RB1 Tumor suppressor involved in Various abnormalities lead 51%
cell cycle control to dysregulated cell cycle
progression
p16 (CDKN2A) Endogenous CDK inhibitors Various abnormalities 40% Poor prognosis
p15 (CDKN2B) (deletion, methylation) 70%
lead to unrestrained cell
cycle progression
TP53 Master regulator of cell cycle, Deletions and inactivating 16% (92% in Poor prognosis; more common in
DNA damage response and point mutations patients with low B-ALL than T-ALL; frequency increases
apoptosis (tumor suppressor) hypodiploidy, 63% with age; “double hit” of TP53 do worst
with MYC translo-
cations, 23% with
complex karyotype)
IKZF1 Transcription factor Splicing abnormalities >80% of patients Poor prognosis independent of Ph
with Ph+ ALL status
“PHILADELPHIA CHROMOSOME LIKE” B-ALL (10-13% of children; 21-27% of adolescents /young adults)
ABL1, ABL2, Various rearrangements Signaling pathway activa- 12.6% Sensitive to Bcr-Abl TKIs, e.g., imatinib,
CSF1R, involving different fusion tion (CRKL phosphorylation dasatinib
PDGFRB partners, e.g., EBF1-PDGFRB, seen with fusions involving
NUP214-ABL1 ABL1/2)
EPOR Various rearrangements Activation of JAK-STAT 3.9% Sensitive to JAK1/2 inhibitor ruxolitinib
involving different fusion signaling
partners, e.g., IGH-EPOR
JAK2 Various rearrangements Activation of JAK-STAT 7.4% Sensitive to JAK1/2 inhibitor ruxolitinib
involving different fusion signaling
partners, e.g., PAX5-JAK2,
ATF7IP-JAK2, BCR-JAK2,
STRB3-JAK2
CRLF2 Various rearrangements Activation of JAK-STAT 49.7% 55% have concomitant JAK1/2 muta-
involving different fusion signaling tion; sensitive to ruxolitinib even with-
partners, e.g., P2RY8-CRLF2, out concomitant JAK mutations
IGH-CRLF2
IL7R, FLT3, Various alterations Activation of JAK-STAT 12.6% Role for therapeutic JAK inhibition?
SH2B3 (LNK), signaling
JAK1, JAK3,
TYK2, IL2RB
Ras/Raf/ Major cellular pathway that Activating mutations 4.3% MEK inhibitors?
MAPK path- controls proliferation, differ-
way genes entiation, transcription regu-
lation and development
(continued)
Kaushansky_chapter 16_p0213-0246.indd 232 9/18/15 11:57 PM