Page 74 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 74
CHaPter 4 Antigen Receptor Genes, Gene Products, and Coreceptors 59
to the antigen to a cell that will respond to antigen with the
production of antibody (Chapter 7).
TCR αβ AND γδ
TCR α, β γ, and δ chains are members of the IgSF and thus Cβ
share a number of structural similarities with Igs. Each chain
contains a leader peptide and extracellular, transmembrane, and Cα
intracytoplasmic components. The extracellular component can
be divided into three domains: a polymorphic V domain encoded
by VJ (α and γ chains) or VDJ (β and δ chains) gene segments,
17
a C domain, and a hinge region. The hinge region typically
contains an extra cysteine (none in γ chains encoded by Cγ2)
that forms a disulfide bond with the other partner of the het-
erodimer. All of the transmembrane domains include a lysine
plus or minus an arginine residue that facilitates the association
of the TCR heterodimer with components of the CD3 signal
transduction complex, each of which has a matching negatively Vα
charged residue in its own transmembrane portion (see below). Vβ
The intracytoplasmic components are tiny and play a minimal
role in signal transduction.
TCR αβ
The TCR α and β chains are glycoproteins with molecular weights
that vary from 42 to 45 kDa, depending on the primary amino
acid sequence and the degree of glycosylation. Deglycosylated
forms have a molecular mass of 30 to 32 kDa. These chains share P8 α 1
a number of invariant residues in common with Ig heavy and P1
light chains, in particular residues that are thought to be important
for interactions between heavy and light chains. The structures
of over 30 partial or full length TCRs have been solved by X-ray α 2
18
crystallography (Fig. 4.3). In general, the structure of the TCR
αβ heterodimer is similar, but not identical, to that of an Ig Fab
fragment.
TCR γδ
The TCR γ and δ chains are glycoproteins with a more complex β m
molecular size pattern than α and β chains. TCRs that use the Cγ1 2
gene segment, which contains a cysteine-encoding exon, are
disulfide-linked (MW 36–42 kDa). TCRs that use Cγ2 exist in two
19
non–disulfide-linked forms, one of 40–44 kDa and one of 55 kDa.
The differences in molecular size result from the variability of both α 3
N-linked glycosylation and primary amino acid sequence. The
55-kDa form uses a Cγ2 allele that contains three (rather than two)
exons encoding the connecting piece, as well as more N-linked
carbohydrate. The TCR δ chain is more straightforward, being
40–43 kDa in size and containing two sites of N-linked glycosylation.
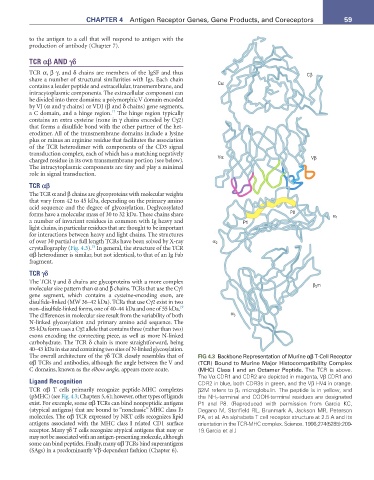
The overall architecture of the γδ TCR closely resembles that of FIG 4.3 Backbone Representation of Murine αβ T-Cell Receptor
αβ TCRs and antibodies, although the angle between the V and (TCR) Bound to Murine Major Histocompatibility Complex
C domains, known as the elbow angle, appears more acute. (MHC) Class I and an Octamer Peptide. The TCR is above.
The Vα CDR1 and CDR2 are depicted in magenta, Vβ CDR1 and
Ligand Recognition CDR2 in blue, both CDR3s in green, and the Vβ HV4 in orange.
TCR αβ T cells primarily recognize peptide-MHC complexes β2M refers to β 2 microglobulin. The peptide is in yellow, and
(pMHC) (see Fig. 4.3; Chapters 5, 6); however, other types of ligands the NH 2-terminal and COOH-terminal residues are designated
exist. For example, some αβ TCRs can bind nonpeptidic antigens P1 and P8. (Reproduced with permission from Garcia KC,
(atypical antigens) that are bound to “nonclassic” MHC class Ib Degano M, Stanfield RL, Brunmark A, Jackson MR, Peterson
molecules. The αβ TCR expressed by NKT cells recognizes lipid PA, et al. An alphabeta T cell receptor structure at 2.5 A and its
antigens associated with the MHC class I related CD1 surface orientation in the TCR-MHC complex. Science. 1996;274(5285):209-
receptor. Many γδ T cells recognize atypical antigens that may or 19.Garcia et al.)
may not be associated with an antigen-presenting molecule, although
some can bind peptides. Finally, many αβ TCRs bind superantigens
(SAgs) in a predominantly Vβ-dependent fashion (Chapter 6).