Page 310 - 9780077418427.pdf
P. 310
/Users/user-f465/Desktop
tiL12214_ch11_275-298.indd Page 287 9/3/10 6:12 PM user-f465
tiL12214_ch11_275-298.indd Page 287 9/3/10 6:12 PM user-f465 /Users/user-f465/Desktop
TABLE 11.2
Some common acids
Name Formula Comment
Acetic acid CH 3 COOH A weak acid found in
vinegar
Boric acid H 3 BO 3 A weak acid used in
eyedrops
Carbonic acid H 2 CO 3 The weak acid of
carbonated beverages
Formic acid HCOOH Makes the sting of some
insects and certain plants
Hydrochloric acid HCl Also called muriatic acid;
used in swimming pools,
soil acidifiers, and stain
removers A
Lactic acid CH 3 CHOHCOOH Found in sour milk,
sauerkraut, and pickles;
gives tart taste to yogurt
Nitric acid HNO 3 A strong acid
Phosphoric acid H 3 PO 4 Used in cleaning solutions;
added to carbonated
beverages for tartness
Sulfuric acid H 2 SO 4 Also called oil of vitriol;
used as battery acid and
in swimming pools
TABLE 11.3
Some common bases
Name Formula Comment B
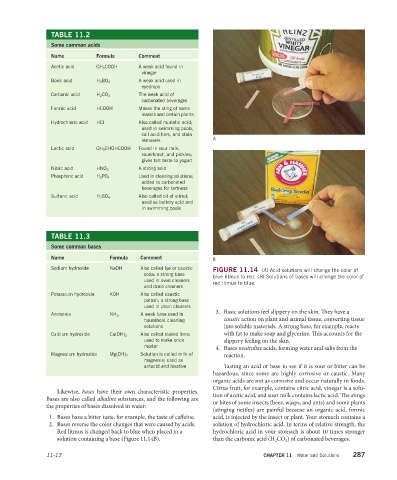
Sodium hydroxide NaOH Also called lye or caustic FIGURE 11.14 (A) Acid solutions will change the color of
soda; a strong base blue litmus to red. (B) Solutions of bases will change the color of
used in oven cleaners red litmus to blue.
and drain cleaners
Potassium hydroxide KOH Also called caustic
potash; a strong base
used in drain cleaners
3. Basic solutions feel slippery on the skin. They have a
Ammonia NH 3 A weak base used in
household cleaning caustic action on plant and animal tissue, converting tissue
solutions into soluble materials. A strong base, for example, reacts
Calcium hydroxide Ca(OH) 2 Also called slaked lime; with fat to make soap and glycerine. This accounts for the
used to make brick slippery feeling on the skin.
mortar 4. Bases neutralize acids, forming water and salts from the
Magnesium hydroxide Mg(OH) 2 Solution is called milk of reaction.
magnesia; used as
antacid and laxative Tasting an acid or base to see if it is sour or bitter can be
hazardous, since some are highly corrosive or caustic. Many
organic acids are not as corrosive and occur naturally in foods.
Citrus fruit, for example, contains citric acid, vinegar is a solu-
Likewise, bases have their own characteristic properties.
tion of acetic acid, and sour milk contains lactic acid. The stings
Bases are also called alkaline substances, and the following are
or bites of some insects (bees, wasps, and ants) and some plants
the properties of bases dissolved in water:
(stinging nettles) are painful because an organic acid, formic
1. Bases have a bitter taste, for example, the taste of caff eine. acid, is injected by the insect or plant. Your stomach contains a
2. Bases reverse the color changes that were caused by acids. solution of hydrochloric acid. In terms of relative strength, the
Red litmus is changed back to blue when placed in a hydrochloric acid in your stomach is about 10 times stronger
solution containing a base (Figure 11.14B). than the carbonic acid (H 2 CO 3 ) of carbonated beverages.
11-13 CHAPTER 11 Water and Solutions 287