Page 348 - 9780077418427.pdf
P. 348
/Users/user-f465/Desktop
tiL12214_ch13_323-350.indd Page 325 9/3/10 6:14 PM user-f465
tiL12214_ch13_323-350.indd Page 325 9/3/10 6:14 PM user-f465 /Users/user-f465/Desktop
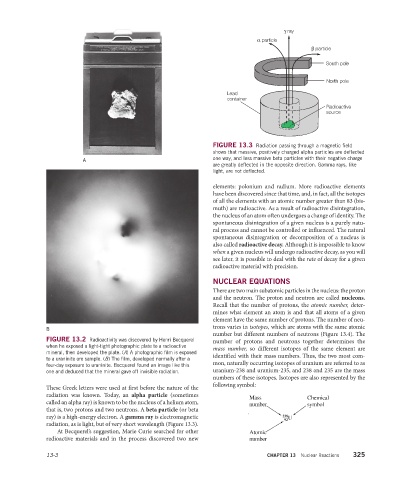
γ ray
α particle
β particle
South pole
North pole
Lead
container
Radioactive
source
FIGURE 13.3 Radiation passing through a magnetic field
shows that massive, positively charged alpha particles are deflected
one way, and less massive beta particles with their negative charge
A
are greatly deflected in the opposite direction. Gamma rays, like
light, are not deflected.
elements: polonium and radium. More radioactive elements
have been discovered since that time, and, in fact, all the isotopes
of all the elements with an atomic number greater than 83 (bis-
muth) are radioactive. As a result of radioactive disintegration,
the nucleus of an atom often undergoes a change of identity. The
spontaneous disintegration of a given nucleus is a purely natu-
ral process and cannot be controlled or influenced. The natural
spontaneous disintegration or decomposition of a nucleus is
also called radioactive decay. Although it is impossible to know
when a given nucleus will undergo radioactive decay, as you will
see later, it is possible to deal with the rate of decay for a given
radioactive material with precision.
NUCLEAR EQUATIONS
There are two main subatomic particles in the nucleus: the proton
and the neutron. The proton and neutron are called nucleons.
Recall that the number of protons, the atomic number, deter-
mines what element an atom is and that all atoms of a given
element have the same number of protons. The number of neu-
trons varies in isotopes, which are atoms with the same atomic
B
number but different numbers of neutrons (Figure 13.4). The
FIGURE 13.2 Radioactivity was discovered by Henri Becquerel number of protons and neutrons together determines the
when he exposed a light-tight photographic plate to a radioactive mass number, so different isotopes of the same element are
mineral, then developed the plate. (A) A photographic film is exposed
identified with their mass numbers. Thus, the two most com-
to a uraninite ore sample. (B) The film, developed normally after a
four-day exposure to uraninite. Becquerel found an image like this mon, naturally occurring isotopes of uranium are referred to as
one and deduced that the mineral gave off invisible radiation. uranium-238 and uranium-235, and 238 and 235 are the mass
numbers of these isotopes. Isotopes are also represented by the
following symbol:
These Greek letters were used at first before the nature of the
radiation was known. Today, an alpha particle (sometimes
called an alpha ray) is known to be the nucleus of a helium atom,
that is, two protons and two neutrons. A beta particle (or beta
ray) is a high-energy electron. A gamma ray is electromagnetic
radiation, as is light, but of very short wavelength (Figure 13.3).
At Becquerel’s suggestion, Marie Curie searched for other
radioactive materials and in the process discovered two new
13-3 CHAPTER 13 Nuclear Reactions 325