Page 350 - 9780077418427.pdf
P. 350
/Users/user-f465/Desktop
tiL12214_ch13_323-350.indd Page 327 9/3/10 6:14 PM user-f465
tiL12214_ch13_323-350.indd Page 327 9/3/10 6:14 PM user-f465 /Users/user-f465/Desktop
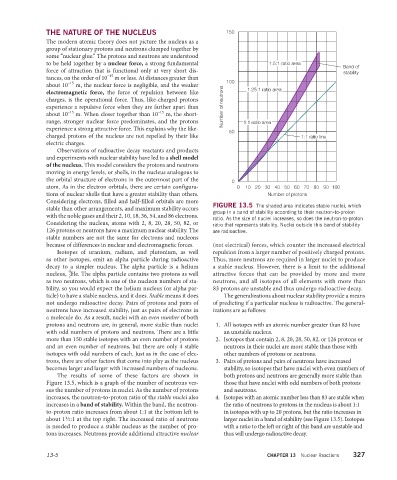
THE NATURE OF THE NUCLEUS 150
The modern atomic theory does not picture the nucleus as a
group of stationary protons and neutrons clumped together by
some “nuclear glue.” The protons and neutrons are understood
to be held together by a nuclear force, a strong fundamental 1.5:1 ratio area Band of
force of attraction that is functional only at very short dis- stability
–15
tances, on the order of 10 m or less. At distances greater than 100
–15
about 10 m, the nuclear force is negligible, and the weaker
electromagnetic force, the force of repulsion between like 1.25:1 ratio area
charges, is the operational force. Thus, like-charged protons
experience a repulsive force when they are farther apart than Number of neutrons
–15
–15
about 10 m. When closer together than 10 m, the short-
range, stronger nuclear force predominates, and the protons 1:1 ratio area
experience a strong attractive force. This explains why the like- 50
charged protons of the nucleus are not repelled by their like 1:1 ratio line
electric charges.
Observations of radioactive decay reactants and products
and experiments with nuclear stability have led to a shell model
of the nucleus. This model considers the protons and neutrons
moving in energy levels, or shells, in the nucleus analogous to
the orbital structure of electrons in the outermost part of the 0
atom. As in the electron orbitals, there are certain configura- 0 10 20 30 40 50 60 70 80 90 100
tions of nuclear shells that have a greater stability than others. Number of protons
Considering electrons, filled and half-filled orbitals are more
stable than other arrangements, and maximum stability occurs FIGURE 13.5 The shaded area indicates stable nuclei, which
group in a band of stability according to their neutron-to-proton
with the noble gases and their 2, 10, 18, 36, 54, and 86 electrons. ratio. As the size of nuclei increases, so does the neutron-to-proton
Considering the nucleus, atoms with 2, 8, 20, 28, 50, 82, or ratio that represents stability. Nuclei outside this band of stability
126 protons or neutrons have a maximum nuclear stability. The are radioactive.
stable numbers are not the same for electrons and nucleons
because of differences in nuclear and electromagnetic forces. (not electrical) forces, which counter the increased electrical
Isotopes of uranium, radium, and plutonium, as well repulsion from a larger number of positively charged protons.
as other isotopes, emit an alpha particle during radioactive Thus, more neutrons are required in larger nuclei to produce
decay to a simpler nucleus. The alpha particle is a helium a stable nucleus. However, there is a limit to the additional
4
nucleus, He. The alpha particle contains two protons as well attractive forces that can be provided by more and more
2
as two neutrons, which is one of the nucleon numbers of sta- neutrons, and all isotopes of all elements with more than
bility, so you would expect the helium nucleus (or alpha par- 83 protons are unstable and thus undergo radioactive decay.
ticle) to have a stable nucleus, and it does. Stable means it does The generalizations about nuclear stability provide a means
not undergo radioactive decay. Pairs of protons and pairs of of predicting if a particular nucleus is radioactive. The general-
neutrons have increased stability, just as pairs of electrons in izations are as follows:
a molecule do. As a result, nuclei with an even number of both
protons and neutrons are, in general, more stable than nuclei 1. All isotopes with an atomic number greater than 83 have
with odd numbers of protons and neutrons. There are a little an unstable nucleus.
more than 150 stable isotopes with an even number of protons 2. Isotopes that contain 2, 8, 20, 28, 50, 82, or 126 protons or
and an even number of neutrons, but there are only 4 stable neutrons in their nuclei are more stable than those with
isotopes with odd numbers of each. Just as in the case of elec- other numbers of protons or neutrons.
trons, there are other factors that come into play as the nucleus 3. Pairs of protons and pairs of neutrons have increased
becomes larger and larger with increased numbers of nucleons. stability, so isotopes that have nuclei with even numbers of
The results of some of these factors are shown in both protons and neutrons are generally more stable than
Figure 13.5, which is a graph of the number of neutrons ver- those that have nuclei with odd numbers of both protons
sus the number of protons in nuclei. As the number of protons and neutrons.
increases, the neutron-to-proton ratio of the stable nuclei also 4. Isotopes with an atomic number less than 83 are stable when
increases in a band of stability. Within the band, the neutron- the ratio of neutrons to protons in the nucleus is about 1:1
to-proton ratio increases from about 1:1 at the bottom left to in isotopes with up to 20 protons, but the ratio increases in
1
about 1 ⁄2:1 at the top right. The increased ratio of neutrons larger nuclei in a band of stability (see Figure 13.5). Isotopes
is needed to produce a stable nucleus as the number of pro- with a ratio to the left or right of this band are unstable and
tons increases. Neutrons provide additional attractive nuclear thus will undergo radioactive decay.
13-5 CHAPTER 13 Nuclear Reactions 327