Page 352 - 9780077418427.pdf
P. 352
/Users/user-f465/Desktop
tiL12214_ch13_323-350.indd Page 329 9/3/10 6:14 PM user-f465
tiL12214_ch13_323-350.indd Page 329 9/3/10 6:14 PM user-f465 /Users/user-f465/Desktop
state of stability, and other isotopes above, below, or beyond the trends, there are exceptions to the summarized relationships
band are unstable and thus radioactive. between neutron-to-proton ratios and radioactive decay.
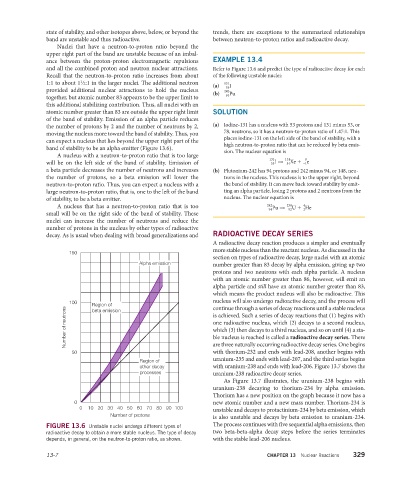
Nuclei that have a neutron-to-proton ratio beyond the
upper right part of the band are unstable because of an imbal-
ance between the proton-proton electromagnetic repulsions EXAMPLE 13.4
and all the combined proton and neutron nuclear attractions. Refer to Figure 13.6 and predict the type of radioactive decay for each
Recall that the neutron-to-proton ratio increases from about of the following unstable nuclei:
1
1:1 to about 1 ⁄2:1 in the larger nuclei. The additional neutron 131
(a) I
53
provided additional nuclear attractions to hold the nucleus 242
(b) Pu
94
together, but atomic number 83 appears to be the upper limit to
this additional stabilizing contribution. Thus, all nuclei with an
atomic number greater than 83 are outside the upper right limit SOLUTION
of the band of stability. Emission of an alpha particle reduces
the number of protons by 2 and the number of neutrons by 2, (a) Iodine-131 has a nucleus with 53 protons and 131 minus 53, or
78, neutrons, so it has a neutron-to-proton ratio of 1.47:1. This
moving the nucleus more toward the band of stability. Thus, you
places iodine-131 on the left side of the band of stability, with a
can expect a nucleus that lies beyond the upper right part of the
high neutron-to-proton ratio that can be reduced by beta emis-
band of stability to be an alpha emitter (Figure 13.6).
sion. The nuclear equation is
A nucleus with a neutron-to-proton ratio that is too large
131
131
0
will be on the left side of the band of stability. Emission of I → Xe + e
54
–1
53
a beta particle decreases the number of neutrons and increases (b) Plutonium-242 has 94 protons and 242 minus 94, or 148, neu-
the number of protons, so a beta emission will lower the trons in the nucleus. This nucleus is to the upper right, beyond
neutron-to-proton ratio. Thus, you can expect a nucleus with a the band of stability. It can move back toward stability by emit-
large neutron-to-proton ratio, that is, one to the left of the band ting an alpha particle, losing 2 protons and 2 neutrons from the
of stability, to be a beta emitter. nucleus. The nuclear equation is
A nucleus that has a neutron-to-proton ratio that is too 242 238 4 2
Pu → U + He
92
94
small will be on the right side of the band of stability. These
nuclei can increase the number of neutrons and reduce the
number of protons in the nucleus by other types of radioactive
decay. As is usual when dealing with broad generalizations and RADIOACTIVE DECAY SERIES
A radioactive decay reaction produces a simpler and eventually
150 more stable nucleus than the reactant nucleus. As discussed in the
section on types of radioactive decay, large nuclei with an atomic
Alpha emission number greater than 83 decay by alpha emission, giving up two
protons and two neutrons with each alpha particle. A nucleus
with an atomic number greater than 86, however, will emit an
alpha particle and still have an atomic number greater than 83,
which means the product nucleus will also be radioactive. This
100 Region of nucleus will also undergo radioactive decay, and the process will
continue through a series of decay reactions until a stable nucleus
Number of neutrons is achieved. Such a series of decay reactions that (1) begins with
beta emission
one radioactive nucleus, which (2) decays to a second nucleus,
which (3) then decays to a third nucleus, and so on until (4) a sta-
ble nucleus is reached is called a radioactive decay series. There
with thorium-232 and ends with lead-208, another begins with
50 are three naturally occurring radioactive decay series. One begins
Region of uranium-235 and ends with lead-207, and the third series begins
other decay with uranium-238 and ends with lead-206. Figure 13.7 shows the
processes uranium-238 radioactive decay series.
As Figure 13.7 illustrates, the uranium-238 begins with
uranium-238 decaying to thorium-234 by alpha emission.
Thorium has a new position on the graph because it now has a
0 new atomic number and a new mass number. Thorium-234 is
0 10 20 30 40 50 60 70 80 90 100 unstable and decays to protactinium-234 by beta emission, which
Number of protons is also unstable and decays by beta emission to uranium-234.
The process continues with five sequential alpha emissions, then
FIGURE 13.6 Unstable nuclei undergo different types of
radioactive decay to obtain a more stable nucleus. The type of decay two beta-beta-alpha decay steps before the series terminates
depends, in general, on the neutron-to-proton ratio, as shown. with the stable lead-206 nucleus.
13-7 CHAPTER 13 Nuclear Reactions 329