Page 353 - 9780077418427.pdf
P. 353
/Users/user-f465/Desktop
tiL12214_ch13_323-350.indd Page 330 9/3/10 6:14 PM user-f465
tiL12214_ch13_323-350.indd Page 330 9/3/10 6:14 PM user-f465 /Users/user-f465/Desktop
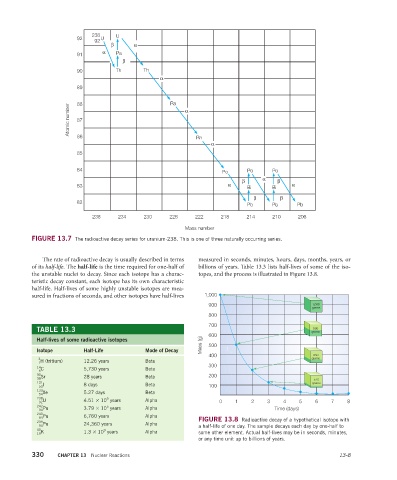
238 U
92 92 U
β α
α Pa
91
β
90 Th Th
α
89 Ra
Atomic number 88 α
87
86
Rn
α
85
84 Po Po Po
β α β
83 α Bi Bi α
β β
82 Pb Pb Pb
238 234 230 226 222 218 214 210 206
Mass number
FIGURE 13.7 The radioactive decay series for uranium-238. This is one of three naturally occurring series.
The rate of radioactive decay is usually described in terms measured in seconds, minutes, hours, days, months, years, or
of its half-life. The half-life is the time required for one-half of billions of years. Table 13.3 lists half-lives of some of the iso-
the unstable nuclei to decay. Since each isotope has a charac- topes, and the process is illustrated in Figure 13.8.
teristic decay constant, each isotope has its own characteristic
half-life. Half-lives of some highly unstable isotopes are mea-
sured in fractions of seconds, and other isotopes have half-lives 1,000
900 1,000
grams
800
700
500
TABLE 13.3 grams
600
Mass (g) 500
Half-lives of some radioactive isotopes
Isotope Half-Life Mode of Decay
400 250
3
H (tritium) 12.26 years Beta grams
1
14 5,730 years Beta 300
C
6
90
Sr 28 years Beta 200 125
38
131
I 8 days Beta 100 grams
53
133
Xe 5.27 days Beta
54
238 9
U 4.51 × 10 years Alpha 0 1 2 3 4 5 6 7 8
92
242 5
Pu 3.79 × 10 years Alpha Time (days)
94
240
Pu 6,760 years Alpha
94
239 FIGURE 13.8 Radioactive decay of a hypothetical isotope with
Pu 24,360 years Alpha a half-life of one day. The sample decays each day by one-half to
94
40 9
K 1.3 × 10 years Alpha some other element. Actual half-lives may be in seconds, minutes,
19
or any time unit up to billions of years.
330 CHAPTER 13 Nuclear Reactions 13-8