Page 358 - 9780077418427.pdf
P. 358
/Users/user-f465/Desktop
tiL12214_ch13_323-350.indd Page 335 9/3/10 6:14 PM user-f465
tiL12214_ch13_323-350.indd Page 335 9/3/10 6:14 PM user-f465 /Users/user-f465/Desktop
A Closer Look
Radiation and Food Preservation
adiation can be used to delay food the nuclei of atoms in food molecules, so it In the United States, the Food and
R spoilage and preserve foods by kill- does not produce radioactivity either. Drug Administration (FDA) regulates which
ing bacteria and other pathogens, just as In addition to killing the parasites and prod ucts can be treated by radiation and
heat is used to pasteurize milk. Foods such bacteria, the process might result in some the dosages used in the treatment. The
as wheat, flour, fruits, vegetables, pork, nutritional loss but no more than that U.S. Department of Agriculture (USDA) is
chicken, turkey, ground beef, and other which normally occurs in canning. Some responsible for the inspection of irradi-
uncooked meats are exposed to gamma new chemical products may be formed ated meat and poultry products. All foods
radiation from cobalt-60 or cesium-137 iso- by the exposure to radiation, but studies that have undergone a radiation treatment
topes, X rays, and electron beams. This kills in several countries have not been able to must show the international logo for this
insects, parasites such as Trichinella spiralis identify any health problems or ill effects and a statement. The logo, called a radura,
and tapeworms, and bacte ria such as E. coli, from these compounds. is a stylized flower inside a circle with five
Listeria, Salmonellae, and Staphylococcus. Treatment with radiation works better openings on the top part (Box Figure 13.1).
The overall effect is that many food-borne for some foods than others. Dairy products
causes of human disease are eliminated, and undergo some flavor changes that are unde-
it is possible to store foods longer. sirable, and some fruits such as peaches
Food in the raw state, processed, or fro- become soft. Irradiated strawberries, on
zen is passed through a machine where it is the other hand, remain firm and last for
irradiated while cold or frozen. This process weeks instead of a few days in the refrigera-
does not make the food radioactive because tor. Foods that are sterilized with a stronger
the food does not touch any radioactive dose of radiation can be stored for years
substance. In addition, the radiation used in without refrigeration just as canned foods
the process is not strong enough to disrupt that have undergone heat pasteurization. BOX FIGURE 13.1
This change in mass is related to the energy change according Iron-56
to the relationship that was formulated by Albert Einstein in
1905. The relationship is 14.0
E = mc 2
equation 13.1 12.0
where E is a quantity of energy, m is a quantity of mass, and c is 10.0 Fission
8
a constant equal to the speed of light in a vacuum, 3.00 × 10 m/s. Binding energy/nucleon (10 –13 J) 8.0
According to this relationship, matter and energy are the same
thing, and energy can be changed to matter and vice versa. 6.0
The products of a mole of uranium-238 decaying to more Fusion
11
stable products (1) have a lower energy of 4.14 × 10 J and 4.0
–6
(2) lost a mass of 4.6 × 10 kg. As you can see, a very small 2.0
amount of matter was converted into a large amount of energy
in the process, forming products of lower energy. 0
The relationship between mass and energy explains why 50 100 150 200 250
the mass of a nucleus is always less than the sum of the masses Mass number
of the individual particles of which it is made. The difference
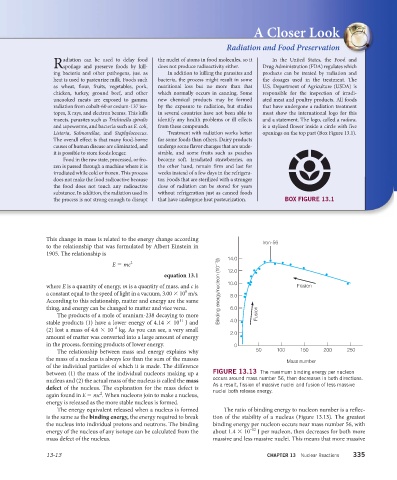
between (1) the mass of the individual nucleons making up a FIGURE 13.13 The maximum binding energy per nucleon
nucleus and (2) the actual mass of the nucleus is called the mass occurs around mass number 56, then decreases in both directions.
As a result, fission of massive nuclei and fusion of less massive
defect of the nucleus. The explanation for the mass defect is nuclei both release energy.
2
again found in E = mc . When nucleons join to make a nucleus,
energy is released as the more stable nucleus is formed.
The energy equivalent released when a nucleus is formed The ratio of binding energy to nucleon number is a reflec-
is the same as the binding energy, the energy required to break tion of the stability of a nucleus (Figure 13.13). The greatest
the nucleus into individual protons and neutrons. The binding binding energy per nucleon occurs near mass number 56, with
–12
energy of the nucleus of any isotope can be calculated from the about 1.4 × 10 J per nucleon, then decreases for both more
mass defect of the nucleus. massive and less massive nuclei. This means that more massive
13-13 CHAPTER 13 Nuclear Reactions 335