Page 20 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 20
Form
4
Chemistry Chapter 5 Chemical Bond
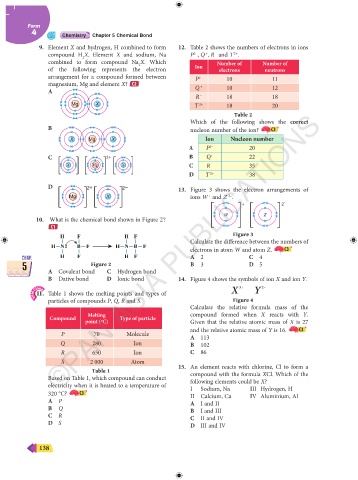
9. Element X and hydrogen, H combined to form 12. Table 2 shows the numbers of electrons in ions
compound H X. Element X and sodium, Na P , Q , R and T .
2–
+
–
2+
2
combined to form compound Na X. Which Number of Number of
2
of the following represents the electron Ion electrons neutrons
arrangement for a compound formed between P 2– 10 11
magnesium, Mg and element X? C2 Q + 10 12
A
R – 18 18
Mg X T 2+ 18 20
Table 2
©PAN ASIA PUBLICATIONS
Which of the following shows the correct
B nucleon number of the ion? C3
X Mg X Ion Nucleon number
A P 2– 20
C – 2+ – B Q + 22
X Mg X C R – 35
D T 2+ 38
D 2+ 2– 13. Figure 3 shows the electron arrangements of
+
2–
Mg X ions W and Z .
+ 2 –
W Z
10. What is the chemical bond shown in Figure 2?
C1
H F H F Figure 3
H N B F H N B F Calculate the difference between the numbers of
electrons in atom W and atom Z. C3
CHAP. H F H F A 2 C 4 CHAP.
Figure 2
5 A Covalent bond C Hydrogen bond B 3 D 5 5
B Dative bond D Ionic bond 14. Figure 4 shows the symbols of ion X and ion Y.
3+
SPM Clone X Y 2–
11. Table 1 shows the melting points and types of
particles of compounds P, Q, R and S. Figure 4
Calculate the relative formula mass of the
Melting compound formed when X reacts with Y.
Compound Type of particle
point ( C) Given that the relative atomic mass of X is 27
o
and the relative atomic mass of Y is 16. C3
P 70 Molecule A 113
Q 280 Ion B 102
R 650 Ion C 86
S 2 000 Atom
Table 1 15. An element reacts with chlorine, Cl to form a
Based on Table 1, which compound can conduct compound with the formula XCl. Which of the
following elements could be X?
electricity when it is heated to a temperature of I Sodium, Na III Hydrogen, H
320 °C? C3 II Calcium, Ca IV Aluminium, Al
A P A I and II
B Q B I and III
C R C II and IV
D S D III and IV
138