Page 17 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 17
Form
4
Chapter 5 Chemical Bond Chemistry
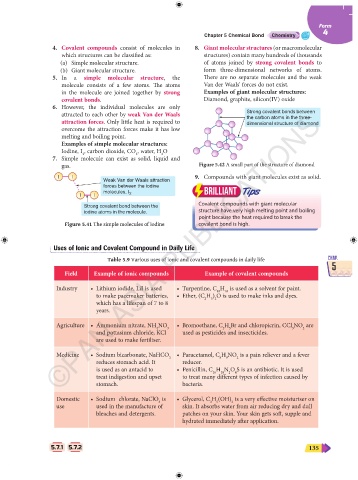
4. Covalent compounds consist of molecules in 8. Giant molecular structures (or macromolecular
which structures can be classified as: structures) contain many hundreds of thousands
(a) Simple molecular structure. of atoms joined by strong covalent bonds to
(b) Giant molecular structure. form three-dimensional networks of atoms.
5. In a simple molecular structure, the There are no separate molecules and the weak
molecule consists of a few atoms. The atoms Van der Waals' forces do not exist.
in the molecule are joined together by strong Examples of giant molecular structures:
covalent bonds. Diamond, graphite, silicon(IV) oxide
6. However, the individual molecules are only
attracted to each other by weak Van der Waals Strong covalent bonds between
©PAN ASIA PUBLICATIONS
attraction forces. Only little heat is required to the carbon atoms in the three-
dimensional structure of diamond
overcome the attraction forces make it has low
melting and boiling point.
Examples of simple molecular structures:
Iodine, I , carbon dioxide, CO , water, H O
2
2
2
7. Simple molecule can exist as solid, liquid and
gas. Figure 5.42 A small part of the structure of diamond
I I 9. Compounds with giant molecules exist as solid.
Weak Van der Waals attraction
forces between the iodine
molecules, I 2
I I
Covalent compounds with giant molecular
Strong covalent bond between the
iodine atoms in the molecule. structure have very high melting point and boiling
point because the heat required to break the
Figure 5.41 The simple molecules of iodine covalent bond is high.
Uses of Ionic and Covalent Compound in Daily Life
CHAP. Table 5.9 Various uses of ionic and covalent compounds in daily life CHAP.
5 5
Field Example of ionic compounds Example of covalent compounds
Industry • Lithium iodide, LiI is used • Turpentine, C H is used as a solvent for paint.
10
18
to make pacemaker batteries, • Ether, (C H ) O is used to make inks and dyes.
2
5 2
which has a lifespan of 7 to 8
years.
Agriculture • Ammonium nitrate, NH NO • Bromoethane, C H Br and chloropicrin, CCl NO are
3
2
3
5
4
2
and pottasium chloride, KCl used as pesticides and insecticides.
are used to make fertiliser.
Medicine • Sodium bicarbonate, NaHCO • Paracetamol, C H NO is a pain reliever and a fever
9
8
3
2
reduces stomach acid. It reducer.
is used as an antacid to • Penicillin, C H N O S is an antibiotic. It is used
16
2
4
18
treat indigestion and upset to treat many different types of infection caused by
stomach. bacteria.
Domestic • Sodium chlorate, NaClO is • Glycerol, C H (OH) is a very effective moisturiser on
3
3
5
3
use used in the manufacture of skin. It absorbs water from air reducing dry and dull
bleaches and detergents. patches on your skin. Your skin gets soft, supple and
hydrated immediately after application.
5.7.1 5.7.2 135