Page 24 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 24
Form
4
Chemistry Chapter 5 Chemical Bond
4. Turn off the switch and heat the M1 until completely melted.
5. Turn on the switch again and observe the brightness of the light bulb. Record the observation in Table 1.
6. Repeat steps 1 to 5 using M2. Record the observations in Table 1.
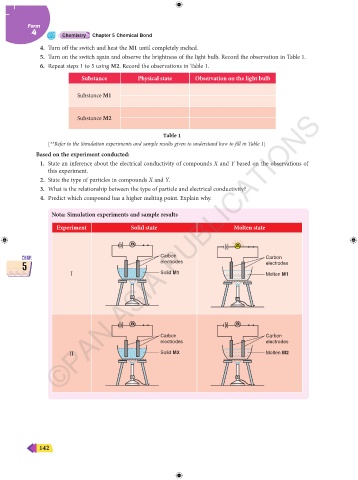
Substance Physical state Observation on the light bulb
Substance M1
Substance M2
Table 1
[**Refer to the Simulation experiments and sample results given to understand how to fill in Table 1]
Based on the experiment conducted:
1. State an inference about the electrical conductivity of compounds X and Y based on the observations of
this experiment.
2. State the type of particles in compounds X and Y.
3. What is the relationship between the type of particle and electrical conductivity?
4. Predict which compound has a higher melting point. Explain why.
Nota: Simulation experiments and sample results
Experiment Solid state Molten state
Carbon
CHAP. ©PAN ASIA PUBLICATIONS CHAP.
Carbon
electrodes
5 electrodes 5
I Solid M1 Molten M1
Carbon Carbon
electrodes electrodes
II Solid M2 Molten M2
142