Page 146 - Basic Principles of Textile Coloration
P. 146
WATER HARDNESS 135
is equivalent to 0.01 mmol Ca2+. Thus, if a 100.0 ml sample of water is titrated
with 0.02 M potassium oleate solution to just give a permanent foam after
addition of V ml of soap, equivalent to V ´ 0.01 mmol Ca2+, the hardness will
be V ´ 10 ppm CaCO3.
Despite the simplicity of this method, it is not always possible to detect
precisely the point at which a layer of foam persists. Hardness is therefore more
usually determined by titration with a standardised solution of the disodium salt of
ethylenediamine tetra-acetic acid (EDTA). EDTA forms very stable, soluble
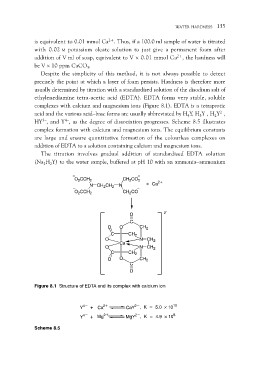
complexes with calcium and magnesium ions (Figure 8.1). EDTA is a tetraprotic
acid and the various acid–base forms are usually abbreviated by H4Y, H3Y–, H2Y2–,
HY3–, and Y4–, as the degree of dissociation progresses. Scheme 8.5 illustrates
complex formation with calcium and magnesium ions. The equilibrium constants
are large and ensure quantitative formation of the colourless complexes on
addition of EDTA to a solution containing calcium and magnesium ions.
The titration involves gradual addition of standardised EDTA solution
(Na2H2Y) to the water sample, buffered at pH 10 with an ammonia–ammonium
O2CCH2 CH2CO2 + Ca2+
N CH2CH2 N
O2CCH2 CH2CO
O 2–
O C
C O CH2
O CH2
Ca N CH2
O
C N CH2
CH2
O O CH2
C
O
Figure 8.1 Structure of EDTA and its complex with calcium ion
Y4 + Ca2 CaY2 , K = 5.0 × 1010
Y4 + Mg2 MgY2 , K = 4.9 × 108
Scheme 8.5