Page 177 - Basic Principles of Textile Coloration
P. 177
166 AUXILIARY CHEMICALS FOR WET PROCESSING AND DYEING
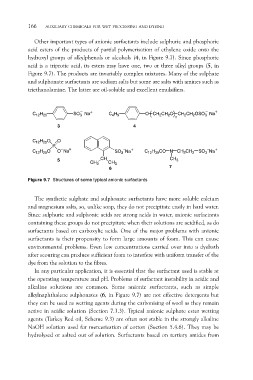
Other important types of anionic surfactants include sulphuric and phosphoric
acid esters of the products of partial polymerisation of ethylene oxide onto the
hydroxyl groups of alkylphenols or alcohols (4, in Figure 9.7). Since phosphoric
acid is a triprotic acid, its esters may have one, two or three alkyl groups (5, in
Figure 9.7). The products are invariably complex mixtures. Many of the sulphate
and sulphonate surfactants are sodium salts but some are salts with amines such as
triethanolamine. The latter are oil-soluble and excellent emulsifiers.
C12H25 SO3 Na C4H9 O CH2CH2O nCH2CH2OSO3 Na
4
3
C12H25O O SO3–Na+ C12H35CO N CH2CH2 SO3–Na+
P CH3
CH 7
C12H25O O Na CH3 CH3
5 6
Figure 9.7 Structures of some typical anionic surfactants
The synthetic sulphate and sulphonate surfactants have more soluble calcium
and magnesium salts, so, unlike soap, they do not precipitate easily in hard water.
Since sulphuric and sulphonic acids are strong acids in water, anionic surfactants
containing these groups do not precipitate when their solutions are acidified, as do
surfactants based on carboxylic acids. One of the major problems with anionic
surfactants is their propensity to form large amounts of foam. This can cause
environmental problems. Even low concentrations carried over into a dyebath
after scouring can produce sufficient foam to interfere with uniform transfer of the
dye from the solution to the fibres.
In any particular application, it is essential that the surfactant used is stable at
the operating temperature and pH. Problems of surfactant instability in acidic and
alkaline solutions are common. Some anionic surfactants, such as simple
alkylnaphthalene sulphonates (6, in Figure 9.7) are not effective detergents but
they can be used as wetting agents during the carbonising of wool as they remain
active in acidic solution (Section 7.3.3). Typical anionic sulphate ester wetting
agents (Turkey Red oil, Scheme 9.3) are often not stable in the strongly alkaline
NaOH solution used for mercerisation of cotton (Section 5.4.6). They may be
hydrolysed or salted out of solution. Surfactants based on tertiary amides from