Page 181 - Basic Principles of Textile Coloration
P. 181
170 AUXILIARY CHEMICALS FOR WET PROCESSING AND DYEING
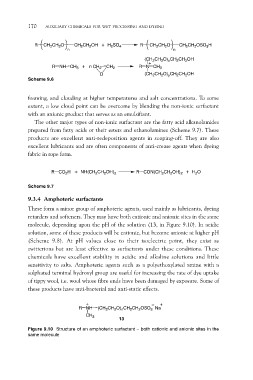
R CH2CH2O CH2CH2OH + H2SO4 R CH2CH2O CH2CH2OSO3H
n n
R NH CH3 + n CH2 CH2 (CH2CH2O)xCH2CH2OH
R N CH3
O
(CH2CH2O)yCH2CH2OH
Scheme 9.6
foaming, and clouding at higher temperatures and salt concentrations. To some
extent, a low cloud point can be overcome by blending the non-ionic surfactant
with an anionic product that serves as an emulsifiant.
The other major types of non-ionic surfactant are the fatty acid alkanolamides
prepared from fatty acids or their esters and ethanolamines (Scheme 9.7). These
products are excellent anti-redeposition agents in soaping-off. They are also
excellent lubricants and are often components of anti-crease agents when dyeing
fabric in rope form.
R CO2H + NH(CH2CH2OH)2 R CON(CH2CH2OH)2 + H2O
Scheme 9.7
9.3.4 Amphoteric surfactants
These form a minor group of amphoteric agents, used mainly as lubricants, dyeing
retarders and softeners. They may have both cationic and anionic sites in the same
molecule, depending upon the pH of the solution (13, in Figure 9.10). In acidic
solution, some of these products will be cationic, but become anionic at higher pH
(Scheme 9.8). At pH values close to their isoelectric point, they exist as
zwitterions but are least effective as surfactants under these conditions. These
chemicals have excellent stability in acidic and alkaline solutions and little
sensitivity to salts. Amphoteric agents such as a polyethoxylated amine with a
sulphated terminal hydroxyl group are useful for increasing the rate of dye uptake
of tippy wool, i.e. wool whose fibre ends have been damaged by exposure. Some of
these products have anti-bacterial and anti-static effects.
R NH (CH2CH2O)nCH2CH2OSO3 Na
CH3 13
Figure 9.10 Structure of an amphoteric surfactant – both cationic and anionic sites in the
same molecule