Page 178 - Basic Principles of Textile Coloration
P. 178
SYNTHETIC SURFACTANTS 167
fatty acids and N-methyltaurine (2-N-methylaminoethane sulphonic acid) are
good detergents with better stability in alkaline solution (7, in Figure 9.7).
Provided that added salts do not precipitate the surfactant by the common ion
effect, they often increase the efficiency of anionic detergents. The incorporation
of cations into the detergent micelles influences the overall charge on the
micelles, the degree to which they repel each other and therefore their emulsifying
action.
9.3.2 Cationic surfactants
These comprise a small group of surfactants that are mainly quaternary
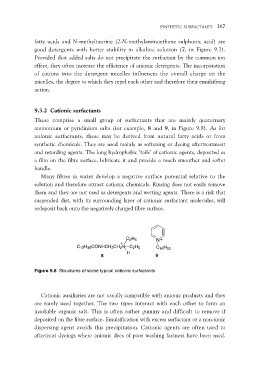
ammonium or pyridinium salts (for example, 8 and 9, in Figure 9.8). As for
anionic surfactants, these may be derived from natural fatty acids or from
synthetic chemicals. They are used mainly as softening or dyeing aftertreatment
and retarding agents. The long hydrophobic ‘tails’ of cationic agents, deposited as
a film on the fibre surface, lubricate it and provide a much smoother and softer
handle.
Many fibres in water develop a negative surface potential relative to the
solution and therefore attract cationic chemicals. Rinsing does not easily remove
them and they are not used as detergents and wetting agents. There is a risk that
suspended dirt, with its surrounding layer of cationic surfactant molecules, will
redeposit back onto the negatively charged fibre surface.
C2H5 N
C16H33
C17H35CONHCH2CH2N C2H5 9
8H
Figure 9.8 Structures of some typical cationic surfactants
Cationic auxiliaries are not usually compatible with anionic products and they
are rarely used together. The two types interact with each other to form an
insoluble organic salt. This is often rather gummy and difficult to remove if
deposited on the fibre surface. Emulsification with excess surfactant or a non-ionic
dispersing agent avoids this precipitation. Cationic agents are often used to
aftertreat dyeings where anionic dyes of poor washing fastness have been used.