Page 411 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 411
CHAPTER 36: Cardiac Arrhythmias, Pacing, Cardioversion, and Defibrillation in the Critical Care Setting 281
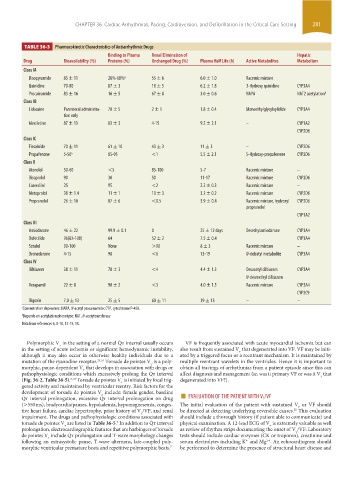
TABLE 36-3 Pharmacokinetic Characteristics of Antiarrhythmic Drugs
Binding to Plasma Renal Elimination of Hepatic
Drug Bioavailability (%) Proteins (%) Unchanged Drug (%) Plasma Half Life (h) Active Metabolites Metabolism
Class IA
Disopyramide 83 ± 11 28%-68% a 55 ± 6 6.0 ± 1.0 Racemic mixture
Quinidine 70-80 87 ± 3 18 ± 5 6.2 ± 1.8 3-Hydroxy quinidine CYP3A4
Procainamide 83 ± 16 16 ± 5 67 ± 8 3.0 ± 0.6 NAPA NAT2 acetylation b
Class IB
Lidocaine Parenteral administra- 70 ± 5 2 ± 1 1.8 ± 0.4 Monoethylglycylxylidide CYP3A4
tion only
Mexiletine 87 ± 13 63 ± 3 4-15 9.2 ± 2.1 – CYP1A2
CYP2D6
Class IC
Flecainide 70 ± 11 61 ± 10 43 ± 3 11 ± 3 – CYP2D6
Propafenone 5-50 a 85-95 <1 5.5 ± 2.1 5-Hydroxy-propafenone CYP2D6
Class II
Atenolol 50-60 <5 85-100 5-7 Racemic mixture –
Bisoprolol 90 30 50 11-17 Racemic mixture CYP2D6
Carvedilol 25 95 <2 2.2 ± 0.3 Racemic mixture –
Metoprolol 38 ± 1.4 11 ± 1 10 ± 3 3.2 ± 0.2 Racemic mixture CYP2D6
Propranolol 26 ± 10 87 ± 6 <0.5 3.9 ± 0.4 Racemic mixture, hydroxyl CYP2D6
propranolol
CYP1A2
Class III
Amiodarone 46 ± 22 99.9 ± 0.1 0 25 ± 12 days Desethylamiodarone CYP3A4
Dofetilide 96(83-108) 64 52 ± 2 7.5 ± 0.4 CYP3A4
Sotalol 90-100 None >90 8 ± 3 Racemic mixture –
Dronedarone 4-15 98 <6 13-19 N-debutyl metabolite CYP3A4
Class IV
Diltiazem 38 ± 11 78 ± 3 <4 4.4 ± 1.3 Desacetyl diltiazem CYP3A4
N-desmethyl diltiazem
Verapamil 22 ± 8 90 ± 2 <3 4.0 ± 1.5 Racemic mixture CYP3A4
CYP2C9
Digoxin 7.0 ± 13 25 ± 5 60 ± 11 39 ± 13 – –
a Concentration dependent: NAPA, N-acetyl procainamide; CYP, cytochrome P-450.
b Depends on acetylation phenotype: NAT, N-acetyltransferase
Data from references 6, 8-10, 12-15, 18.
Polymorphic V in the setting of a normal Q ˙ t interval usually occurs VF is frequently associated with acute myocardial ischemia but can
t
in the setting of acute ischemia or significant hemodynamic instability, also result from sustained V that degenerated into VF. VF may be initi-
t
although it may also occur in otherwise healthy individuals due to a ated by a triggered focus or a reentrant mechanism. It is maintained by
mutation of the ryanodine receptor. 23,24 Torsade de pointes V is a poly- multiple reentrant wavelets in the ventricles. Hence it is important to
t
morphic, pause-dependent V that develops in association with drugs or obtain all tracings of arrhythmias from a patient episode since this can
t
pathophysiologic conditions which excessively prolong the Q ˙ t interval affect diagnosis and management (ie, was it primary VF or was it V that
t
(Fig. 36-2, Table 36-5). Torsade de pointes V is initiated by focal trig- degenerated into VF?).
8,10
t
gered activity and maintained by ventricular reentry. Risk factors for the
Q ˙ t interval prolongation, excessive Q ˙ t interval prolongation on drug ■ EVALUATION OF THE PATIENT WITH V T /VF
development of torsade de pointes V include: female gender, baseline
t
(>550 ms), bradycardia/pauses, hypokalemia, hypomagnesemia, conges- The initial evaluation of the patient with sustained V or VF should
t
tive heart failure, cardiac hypertrophy, prior history of V /VF, and renal be directed at detecting underlying reversible causes. This evaluation
25
t
impairment. The drugs and pathophysiologic conditions associated with should include a thorough history (if patient able to communicate) and
torsade de pointes V are listed in Table 36-5. In addition to Q ˙ t interval physical examination. A 12-lead ECG of V is extremely valuable as well
7
t
t
prolongation, electrocardiographic features that are harbingers of torsade as review of rhythm strips documenting the onset of V /VF. Laboratory
t
de pointes V include Q ˙ t prolongation and T-wave morphology changes tests should include cardiac enzymes (CK or troponin), creatinine and
t
following an extrasystolic pause, T-wave alternans, late-coupled poly- serum electrolytes including K and Mg . An echocardiogram should
+
2+
morphic ventricular premature beats and repetitive polymorphic beats. 7 be performed to determine the presence of structural heart disease and
section03.indd 281 1/23/2015 2:07:09 PM