Page 950 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 950
Chapter 56 Conventional and Molecular Cytogenomic Basis of Hematologic Malignancies 833
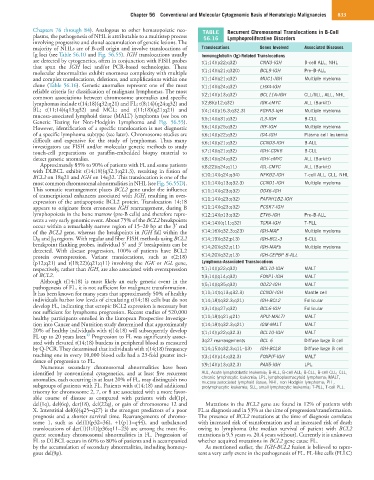
Chapters 76 through 84). Analogous to other hematopoietic neo- TABLE Recurrent Chromosomal Translocations in B-Cell
plasms, the pathogenesis of NHL is attributable to a multistep process 56.16 Lymphoproliferative Disorders
involving progressive and clonal accumulation of genetic lesions. The
majority of NHLs are of B-cell origin and involve translocations of Translocations Genes Involved Associated Diseases
Ig loci (see Table 56.10 and Fig. 56.55). IGH translocations usually Immunoglobulin (Ig)-Related Translocations
are detected by cytogenetics, often in conjunction with FISH probes t(1;14)(p22;q32) CNN3-IGH B-cell ALL, NHL
that span the IGH loci and/or PCR-based technologies. These
molecular abnormalities exhibit enormous complexity with multiple t(1;14)(q21;q320) BCL9-IGH Pre–B-ALL
and complex translocations, deletions, and amplifications within one t(1;14)(q21;q32) MUC1-IGH Multiple myeloma
clone (Table 56.16). Genetic anomalies represent one of the most t(1;14)(q24;q32) LHX4-IGH
reliable criteria for classification of malignant lymphomas. The most
common associations between chromosome anomalies and specific t(2;14)(p13;q32) BCL11A-IGH CLL/SLL, ALL, NHL
lymphomas include t(14;18)(q32;q21) and FL; t(8;14)(q24;q32) and t(2;8)(p12;q32) IGK-cMYC ALL (Burkitt)
BL; t(11;14)(q13;q32) and MCL; and t(11;18)(q21;q21) and t(4;14)(p16.3;q32.3) FGFR3-IqH Multiple myeloma
mucosa-associated lymphoid tissue (MALT) lymphoma (see box on t(5;14)(q31;q32) IL3-IGH B-CLL
Genetic Testing for Non-Hodgkin Lymphoma and Fig. 56.55).
However, identification of a specific translocation is not diagnostic t(6;14)(p25;q32) IRF-IGH Multiple myeloma
of a specific lymphoma subtype (see later). Chromosome studies are t(6;14)(p22;q32) ID4-IGH Plasma cell leukemia
difficult and expensive for the study of lymphomas. Thus many t(6;14)(p21;q32) CCND3-IGH B-ALL
investigators use FISH and/or molecular genetic methods to study
touch-cell preparations or paraffin-embedded biopsy material to t(7;14)(q21;q32) IGH/-CDK6 B-CLL
detect genetic anomalies. t(8;14)(q24;q32) IGH/-cMYC ALL (Burkitt)
Approximately 85% to 90% of patients with FL and some patients t(8;22)(q24;q11) IGL-CMYC ALL (Burkitt)
with DLBCL exhibit t(14;18)(q32.3;q21.3), resulting in fusion of
BCL2 on 18q21 and IGH on 14q32. This translocation is one of the t(10;14)(q24;q34) NFKB2-IGH T-cell ALL, CLL, NHL
most common chromosomal abnormalities in NHL (see Fig. 56.55D). t(11;14)(q13;q32.3) CCND1-IGH Multiple myeloma
This somatic rearrangement places BCL2 gene under the influence t(11;14)(q23;q32) DDX6-IGH
of transcriptional enhancers associated with IGH, resulting in over-
expression of the antiapoptotic BCL2 protein. Translocation 14;18 t(11;14)(q23;q32) PAFAH1B2-IGH
appears to originate from erroneous IGH rearrangement, during B t(11;14)(q23;q32) PCSK7-IGH
lymphopoiesis in the bone marrow (pre-B cells) and therefore repre- t(12;14)(p13;q32) ETV6-IGH Pre–B-ALL
sents a very early genomic event. About 75% of the BCL2 breakpoints t(14;14)((q11;q32) TCRA-IGH T-PLL
occur within a remarkably narrow region of 15–20 bp at the 3′ end
of the BCL2 gene, whereas the breakpoints in IGH fall within the t(14;16)(q32.3;q23) IGH-MAF Multiple myeloma
D H and J H regions. With regular and fiber FISH methods using BCL2 t(14;19)(q32;p13) IGH-BCL-3 B-CLL
breakpoint flanking probes, individual 5′ and 3′ breakpoints can be t(14;20)(q32;q11) IGH-MAFb Multiple myeloma
detected. With disease progression, 100% of patients have BCL2
protein overexpression. Variant translocations, such as t(2;18) t(14;20)(q32;q13) IGH-CEPBP B-ALL
(p12;q21) and t(18;22)(q21;q11) involving the IGK or IGL gene, Lymphoma-Associated Translocations
respectively, rather than IGH, are also associated with overexpression t(1;14)(p22;q32) BCL10-IGH MALT
of BCL2. t(3;14)(q14;q32) FOXP1-IGH MALT
Although t(14;18) is most likely an early genetic event in the
pathogenesis of FL, it is not sufficient for malignant transformation. t(5;14)(q35;q32) ODZ2-IGH MALT
It has been known for many years that approximately 50% of healthy t(11;14)(q13;q32.3) CCNDI-IGH Mantle cell
individuals harbor low levels of circulating t(14;18) cells but do not t(14;18)(q32.3;q21) IGH-BCL2 Follicular
develop FL, indicating that ectopic BCL2 expression is necessary but t(3;14)(q27;q32) BCL6-IGH Follicular
not sufficient for lymphoma progression. Recent studies of 520,000
healthy participants enrolled in the European Prospective Investiga- t(11;18)(q21;q21) API2-MALTI MALT
tion into Cancer and Nutrition study determined that approximately t(14;18)(q32.3;q21) IGM-MALT MALT
20% of healthy individuals with t(14;18) will subsequently develop t(1;14)(p22;q32.3) BCL10-IGH MALT
23
FL up to 20 years later. Progression to FL was significantly associ-
ated with elevated t(14;18) burdens in peripheral blood as measured 3q27 rearrangements BCL 6 Diffuse large B cell
by Q-PCR. They determined that individuals with t(14;18) frequency t(14;15)(q32.3;q11–13) IGH-BCL8 Diffuse large B cell
reaching one in every 10,000 blood cells had a 23-fold greater inci- t(3;14)(p14;q32.3) FOXPIF-IGH MALT
dence of progression to FL.
Numerous secondary chromosomal abnormalities have been t(9;14)(p13;q32.3) PAX5-IGH LPL
identified by conventional cytogenetics, and at least five recurrent ALL, Acute lymphoblastic leukemia; B-ALL, B-cell ALL; B-CLL, B-cell CLL; CLL,
anomalies, each occurring in at least 20% of FL, may distinguish two chronic lymphocytic leukemia; LPL, lymphoplasmacytoid lymphoma; MALT,
mucosa-associated lymphoid tissue; NHL, non-Hodgkin lymphoma; PLL,
subgroups of patients with FL. Patients with t(14;18) and additional prolymphocytic leukemia; SLL, small lymphocytic leukemia; T-PLL, T-cell PLL.
trisomy for chromosome 2, 7, or 8 are associated with a more favor-
able course of disease as compared with patients with del(1p),
del(1q), del(6q), der(18), del(22q), or gain of chromosome 12 and Mutations in the BCL2 gene are found in 12% of patients with
X. Interstitial del(6)(q25–q27) is the strongest predictors of a poor FL at diagnosis and in 53% at the time of progression/transformation.
prognosis and a shorter survival time. Rearrangements of chromo- The presence of BCL2 mutations at the time of diagnosis correlates
some 1, such as del(1)(p32–36), +1(p11–q44), and unbalanced with increased risk of transformation and an increased risk of death
translocations of der(1)(1;1)(p36;q11–23) are among the most fre- owing to lymphoma (the median survival of patient with BCL2
quent secondary chromosomal abnormalities in FL. Progression of mutations is 9.5 years vs. 20.4 years without). Currently it is unknown
FL to DLBCL occurs in 60% to 80% of patients and is accompanied whether acquired mutations in BCL2 gene cause FL.
by the accumulation of secondary abnormalities, including homozy- As mentioned earlier, the IGH-BCL2 fusion is believed to repre-
gous del(9p). sent a very early event in the pathogenesis of FL. FL-like cells (FLLC)