Page 1107 - Williams Hematology ( PDFDrive )
P. 1107
1082 Part VIII: Monocytes and Macrophages Chapter 68: Production, Distribution, and Activation of Monocytes and Macrophages 1083
unique T cell function. The “classical” DC develops from a precursor
which is dependent on the growth factor receptor. 62–64
Spleen
From the macrophage point of view, the spleen is the most complex
organ in the body. 65,66 Our knowledge is based mainly on the mouse
67
and we know there is considerable species variation, as well as con-
stitutive hematopoiesis in mouse spleen. Subpopulations observed
in the mouse by marker and genetic knockout experiments include
(1) macrophages in the red pulp, white pulp, and in the marginal zone,
30
itself M-CSF dependent, and (2) heterogeneous, more phagocytic
“metallophilic” macrophages in the outer marginal zone. Characteristic
A B phenotypic markers are available to identify macrophages in mouse
spleen (see Fig. 68–5C to E). The F4/80 antigen and the mannose
receptor (MR) are restricted to mature macrophages in the red pulp,
whereas CD68 is a marker for all macrophages, as well as DCs, although
the mainly intracellular expression of CD68 is less prominent in DCs.
There are several well-characterized markers for mouse metallophilic
macrophages, including sialoadhesin (Sn), a poorly characterized pro-
tein recognized by the MOMA-1 monoclonal antibody, and ligands for
MR cysteine-rich domain-Fc chimeric proteins (see Fig. 68–5E). The
68
splenic marginal zone macrophage population develops postnatally,
in parallel with antipolysaccharide responses to encapsulated bacteria.
Functions of splenic marginal zone macrophages include clearance of
senescent erythrocytes and neutrophils (red pulp), targeting of circulat-
C D ing antigens and pathogens (marginal zone), interferon (IFN) produc-
tion, induction of secondary adaptive immune responses, regulation of
hematopoiesis, and iron storage. Markers for the outer marginal zone
macrophages include MARCO and SIGNR1, a mouse homologue of
dendritic cell–specific intercellular adhesion molecule-3–grabbing
nonintegrin (DC-SIGN). The spleen is also a site for storage and rapid
deployment of monocytes, which participate in wound healing and play
a role regulation of inflammation. 69
Lymph Nodes
Lymph node macrophages are also heterogeneous, with distinctive
Sn subcapsular cells, corresponding to marginal metallophils in their
marker expression, and F4/80+ macrophages in germinal follicles and
E F in the hilus. Macrophages in T-lymphocyte–rich areas are F4/80– or
dim, as in T-cell areas in spleen, but express CD68. It is thought that
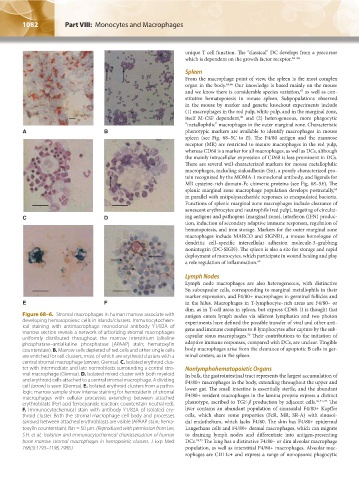
Figure 68–6. Stromal macrophages in human marrow associate with antigen enters lymph nodes via afferent lymphatics and two photon
developing hematopoietic cells in islands/clusters. Immunocytochem- experiments have defined the possible transfer of viral and other anti-
ical staining with antimacrophage monoclonal antibody Y1/82A of gens and immune complexes to B lymphocytes after capture by the sub-
marrow section reveals a network of arborizing stromal macrophages capsular sinus macrophages. Their contributions to the initiation of
70
uniformly distributed throughout the marrow interstitium (alkaline
phosphatase–antialkaline phosphatase [APAAP] stain; hematoxylin adaptive immune responses, compared with DCs, are unclear. Tingible
counterstain). B. Marrow cells depleted of red cells and other single cells body macrophages arise from the clearance of apoptotic B cells in ger-
are enriched for cell clusters, most of which are erythroid clusters with a minal centers, as in the spleen.
central stromal macrophage (arrows; Giemsa). C. Isolated erythroid clus-
ter with intermediate and late normoblasts surrounding a central stro- Nonlymphohematopoietic Organs
mal macrophage (Giemsa). D. Isolated mixed cluster with both myeloid In bulk, the gastrointestinal tract represents the largest accumulation of
and erythroid cells attached to a central stromal macrophage. A dividing F4/80+ macrophages in the body, extending throughout the upper and
cell (arrow) is seen (Giemsa). E. Isolated erythroid clusters from a patho- lower gut. The small intestine is essentially sterile, and the abundant
logic marrow sample show intense staining for hemosiderin of stromal F4/80+ resident macrophages in the lamina propria express a distinct
macrophages with cellular processes extending between attached 56,71–73
erythroblasts (Perl acid ferrocyanide reaction; counterstain neutral red). phenotype, ascribed to TGF-β production by adjacent cells. The
F. Immunocytochemical stain with antibody Y1/82A of isolated ery- liver contains an abundant population of sinusoidal F4/80+ Kupffer
throid cluster. Both the stromal macrophage cell body and processes cells, which share some properties (FcR, MR, SR-A) with sinusoi-
(arrows) between attached erythroblasts are visible (APAAP stain; hema- dal endothelium, which lacks F4/80. The skin has F4/80+ epidermal
toxylin counterstain). Bar = 50 μm. (Reproduced with permission from Lee, Langerhans cells and F4/80+ dermal macrophages, which can migrate
S.H. et al.: Isolation and immunocytochemical characterization of human to draining lymph nodes and differentiate into antigen-presenting
bone marrow stromal macrophages in hemopoietic clusters. J Exp Med DCs. 74,75 The lung has a distinctive F4/80– or dim alveolar macrophage
168(3):1193–1198, 1988.) population, as well as interstitial F4/80+ macrophages. Alveolar mac-
rophages are CD11c+ and express a range of nonopsonic phagocytic
Kaushansky_chapter 68_p1075-1088.indd 1082 9/17/15 3:41 PM