Page 1952 - Williams Hematology ( PDFDrive )
P. 1952
1926 Part XII: Hemostasis and Thrombosis Chapter 113: Molecular Biology and Biochemistry of the Coagulation Factors 1927
Apple 2
EGF 3 EGF 4
Apple 1
EGF 2 Apple 3
EGF 1 SHBG
Apple 4
Catalytic domain
TSR
Gla domain Prepro leader
Protein S Factor XI
Type II
Kunitz 2 Kunitz 3
EGF 2 Kringle
Type I EGF 1
Pro
Kunitz 1
Catalytic domain
Factor XII TFPI`
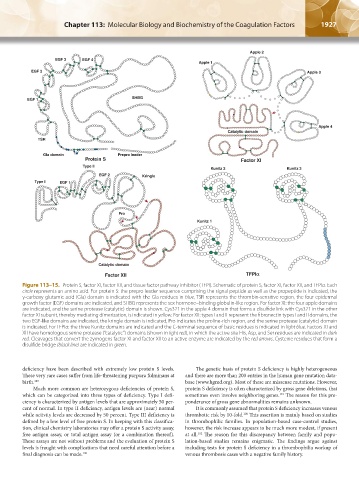
Figure 113–15. Protein S, factor XI, factor XII, and tissue factor pathway inhibitor (TFPI). Schematic of protein S, factor XI, factor XII, and TFPIα. Each
circle represents an amino acid. For protein S: the prepro leader sequence comprising the signal peptide as well as the propeptide is indicated, the
γ-carboxy glutamic acid (Gla) domain is indicated with the Gla residues in blue, TSR represents the thrombin-sensitive region, the four epidermal
growth factor (EGF) domains are indicated, and SHBG represents the sex hormone–binding globulin-like region. For factor XI: the four apple domains
are indicated, and the serine protease (catalytic) domain is shown. Cys321 in the apple 4 domain that forms a disulfide link with Cys321 in the other
factor XI subunit, thereby mediating dimerization, is indicated in yellow. For factor XII: types I and II represent the fibronectin types I and II domains, the
two EGF-like domains are indicated, the kringle domain is indicated, Pro indicates the proline-rich region, and the serine protease (catalytic) domain
is indicated. For TFPIα: the three Kunitz domains are indicated and the C-terminal sequence of basic residues is indicated in light blue. Factors XI and
XII have homologous serine protease (“catalytic”) domains (shown in light red), in which the active site His, Asp, and Ser residues are indicated in dark
red. Cleavages that convert the zymogens factor XI and factor XII to an active enzyme are indicated by the red arrows. Cysteine residues that form a
disulfide bridge (black line) are indicated in green.
deficiency have been described with extremely low protein S levels. The genetic basis of protein S deficiency is highly heterogeneous
These very rare cases suffer from life-threatening purpura fulminans at and there are more than 200 entries in the human gene mutation data-
birth. 189 base (www.hgmd.org). Most of these are missense mutations. However,
Much more common are heterozygous deficiencies of protein S, protein S deficiency is often characterized by gross gene deletions, that
which can be categorized into three types of deficiency. Type I defi- sometimes even involve neighboring genes. The reason for this pre-
191
ciency is characterized by antigen levels that are approximately 50 per- ponderance of gross gene abnormalities remains unknown.
cent of normal. In type II deficiency, antigen levels are (near) normal It is commonly assumed that protein S deficiency increases venous
while activity levels are decreased by 50 percent. Type III deficiency is thrombotic risk by 10-fold. This assertion is mainly based on studies
100
defined by a low level of free protein S. In keeping with this classifica- in thrombophilic families. In population-based case-control studies,
tion, clinical chemistry laboratories may offer a protein S activity assay, however, the risk increase appears to be much more modest, if present
free antigen assay, or total antigen assay (or a combination thereof). at all. The reason for this discrepancy between family and popu-
192
These assays are not without problems and the evaluation of protein S lation-based studies remains enigmatic. The findings argue against
levels is fraught with complications that need careful attention before a including tests for protein S deficiency in a thrombophilia workup of
final diagnosis can be made. 190 venous thrombosis cases with a negative family history.
Kaushansky_chapter 113_p1915-1948.indd 1927 9/21/15 2:39 PM