Page 1297 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1297
CHaPter 93 Assessment of Functional Immune Responses in Lymphocytes 1259
Anti-CD3/CD45 Anti-CD3+anti-CD28/CD45 Anti-CD3+IL-2/CD45
100
60
80
60 Proliferating CD45+ lymphs
Proliferating CD45+ lymphs Proliferating CD45+ lymphs
60 40
Count Count 40 Count
40
20
20
20
0 0 0
0 10 0 10 1 10 2 10 3 0 10 0 10 1 10 2 10 3 0 10 0 10 1 10 2 10 3
Fluorescence Fluorescence Fluorescence
Anti-CD3/CD3 Anti-CD3+anti-CD28/CD3 Anti-CD3+IL-2/CD3
100
60 50
80
Proliferating CD3+ T cells
40
Proliferating CD3+ T cells Proliferating CD3+ T cells
60 40
Count Count Count 30
40
20
20
20
10
0 0 0
0 10 0 10 1 10 2 10 3 0 10 0 10 1 10 2 10 3 0 10 0 10 1 10 2 10 3
Fluorescence Fluorescence Fluorescence
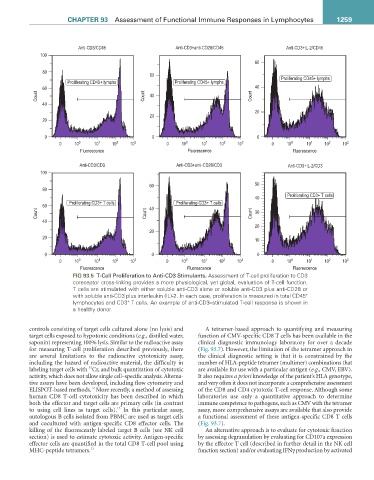
FIG 93.5 T-Cell Proliferation to Anti-CD3 Stimulants. Assessment of T-cell proliferation to CD3
coreceptor cross-linking provides a more physiological, yet global, evaluation of T-cell function.
T cells are stimulated with either soluble anti-CD3 alone or soluble anti-CD3 plus anti-CD28 or
+
with soluble anti-CD3 plus interleukin (IL)-2. In each case, proliferation is measured in total CD45
lymphocytes and CD3 T cells. An example of anti-CD3–stimulated T-cell response is shown in
+
a healthy donor.
controls consisting of target cells cultured alone (no lysis) and A tetramer-based approach to quantifying and measuring
target cells exposed to hypotonic conditions (e.g., distilled water, function of CMV-specific CD8 T cells has been available in the
saponin) representing 100% lysis. Similar to the radioactive assay clinical diagnostic immunology laboratory for over a decade
for measuring T-cell proliferation described previously, there (Fig. 93.7). However, the limitation of the tetramer approach in
are several limitations to the radioactive cytotoxicity assay, the clinical diagnostic setting is that it is constrained by the
including the hazard of radioactive material, the difficulty in number of HLA-peptide tetramer (multimer) combinations that
51
labeling target cells with Cr, and bulk quantitation of cytotoxic are available for use with a particular antigen (e.g., CMV, EBV).
activity, which does not allow single cell–specific analysis. Alterna- It also requires a priori knowledge of the patient’s HLA genotype,
tive assays have been developed, including flow cytometry and and very often it does not incorporate a comprehensive assessment
12
ELISPOT-based methods. More recently, a method of assessing of the CD8 and CD4 cytotoxic T-cell response. Although some
human CD8 T-cell cytotoxicity has been described in which laboratories use only a quantitative approach to determine
both the effector and target cells are primary cells (in contrast immune competence to pathogens, such as CMV with the tetramer
13
to using cell lines as target cells). In this particular assay, assay, more comprehensive assays are available that also provide
autologous B cells isolated from PBMC are used as target cells a functional assessment of these antigen-specific CD8 T cells
and cocultured with antigen-specific CD8 effector cells. The (Fig. 93.7).
killing of the fluorescently labeled target B cells (see NK cell An alternative approach is to evaluate for cytotoxic function
section) is used to estimate cytotoxic activity. Antigen-specific by assessing degranulation by evaluating for CD107a expression
effector cells are quantified in the total CD8 T-cell pool using by the effector T cell (described in further detail in the NK cell
MHC-peptide tetramers. 13 function section) and/or evaluating IFNγ production by activated