Page 1812 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 1812
CHAPTER 129: Dermatologic Conditions 1281
TABLE 129-1 Basic Morphologic and Descriptive Terminology
Macule Flat lesion of variable size
Papule Elevated lesion less than 0.5 cm diameter
Plaque Elevated lesion greater than 0.5 cm diameter
Nodule Elevated, palpable lesion greater than 0.5 cm diameter
Vesicle Fluid filled lesion less than 0.5 cm diameter
Bullae Fluid filled lesion greater than 0.5 cm diameter
Pustule Pus-filled lesion
Ulcer Depressed lesion with loss of epidermis and variable levels of dermis
Wheal Evanescent pale-red papule or plaque
■ EPIDEMIOLOGY OF ADVERSE DRUG REACTIONS
The World Health Organization (WHO) defines an adverse drug reac-
tion (ADR) as any noxious, unintended, and undesired effect of a drug
that occurs at diagnostic, prophylactic, or therapeutic doses used in
humans. This definition excludes untoward events due to noncompli-
1
ance or errors in drug administration, therapeutic failures, intentional
and accidental poisoning, and drug abuse. A meta-analysis of 39 pro-
spective studies covering 32 years reported a 10.9% incidence of ADR
in admitted hospital patients and a 4.7% incidence for patients admitted
because of serious ADR. In addition, fatal ADR ranked “between the
2
fourth and sixth” leading causes of death in the United States in 1994, FIGURE 129-2. Morbilliform drug eruption. (Used with permission of Dr Aisha Sethi.)
exceeding deaths due to pneumonia and diabetes. The rate and severity
2
of preventable ADRs in intensive care units (ICUs) are nearly twice that contacts should be questioned. Table 129-2 outlines the information
in non-ICUs. 3 that must be obtained. With all this information in hand and knowing
Cutaneous ADRs (CADRs) are the most common type of ADR and the reaction rate of various medications, identification of the cause of an
occur in 2% to 3% of hospitalized patients. The numbers of CADR may eruption becomes more likely.
4
be higher in the ICU setting due to the critical and compromised nature Despite the benign nature of the overwhelming majority of CADRs,
of the patient compounded by the multiplicity of drugs. Several factors it is important to evaluate for increasing liver or renal dysfunction and
influence the probability of a drug producing an adverse reaction: the size for signs suggesting progression to severe skin disease (Stevens-Johnson
of the compound (larger compounds are more likely to act as haptens), syndrome or toxic epidermal necrolysis). Signs indicative of serious skin
drug-drug interactions (altered metabolism and protein displacement), problems include mucosal involvement, blistering lesions, and a positive
the route of delivery (intravenous administration increases the incidence Nikolsky sign (Table 129-3).
of reactions), and patient factors such as renal function, alcohol use,
hepatic function, and severity of concomitant disease. Antibiotics ■ CLASSIFICATION OF CUTANEOUS ADVERSE DRUG REACTIONS
5,6
(eg, amoxicillin, penicillin, fluoroquinolones, sulfonamides, and cepha-
losporins) and nonsteroidal anti-inflammatory agents (NSAIDs) are the The most widely used classification scheme for ADR was devised by
5
most likely medications to cause CADR. Antiepileptics (eg, phenytoin Rawlins and Thomson (Table 129-4). Type A reactions are the most
and carbamazepine) are also common causal agents. The following common (80%) and can occur at any dose. Type B reactions occur
medications only rarely cause CADR: digoxin, acetaminophen, meperi-
dine, aminophylline, diphenhydramine, bisacodyl, prochlorperazine,
spironolactone, prednisone, thiamine, ferrous sulfate, atropine, mor-
phine, insulin, and spironolactone.
■ AN APPROACH TO CUTANEOUS ADVERSE DRUG REACTIONS
Cutaneous eruptions are the most frequent ADR in hospitalized
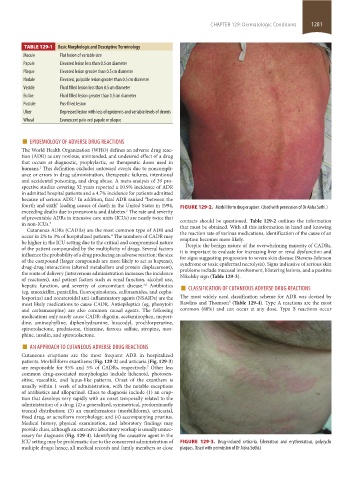
patients. Morbilliform exanthems (Fig. 129-2) and urticaria (Fig. 129-3)
are responsible for 95% and 5% of CADRs, respectively. Other less
7
common drug-associated morphologies include lichenoid, photosen-
sitive, vasculitic, and lupus-like patterns. Onset of the exanthem is
usually within 1 week of administration, with the notable exceptions
of antibiotics and allopurinol. Clues to diagnosis include (1) an erup-
tion that develops very rapidly with an onset temporally related to the
administration of a drug; (2) a generalized, symmetrical, predominantly
truncal distribution; (3) an exanthematous (morbilliform), urticarial,
fixed drug, or acneiform morphology; and (4) accompanying pruritus.
Medical history, physical examination, and laboratory findings may
provide clues, although an extensive laboratory workup is usually unnec-
essary for diagnosis (Fig. 129-4). Identifying the causative agent in the
ICU setting may be problematic due to the concurrent administration of FIGURE 129-3. Drug-induced urticaria. Edematous and erythematous, polycyclic
multiple drugs; hence, all medical records and family members or close plaques. (Used with permission of Dr Aisha Sethi.)
section11.indd 1281 1/19/2015 10:52:39 AM