Page 28 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 28
Form
5
Chapter 1 Redox Equilibrium Chemistry
CHAP. 2. Bromine water, Br acts as the oxidising agent, 5. Iron(III) ions, Fe are reduced to iron(II) ions, CHAP.
3+
1 whereas iron(II) ions, Fe act as the reducing Fe by zinc, Zn. 1
2
2+
2+
3+
agent. 6. Iron(III) ion, Fe act as the oxidising agent
3. An iron(II) ion, Fe loses an electron to form whereas zinc, Zn acts as the reducing agent.
2+
2+
iron(III) ion, Fe . Thus, iron(II) ion, Fe is 7. A zinc atom loses two electrons to form zinc
3+
oxidised to iron(III) ion, Fe . ion, Zn . Thus, zinc atom, Zn is oxidised to
3+
2+
2+
4. A bromine molecule, Br receives two zinc ion, Zn .
2
electrons from iron(II) ion, Fe to form 8. An iron(III) ion, Fe receives an electron from
3+
2+
2+
–
bromide ions, Br . Thus, bromine molecule, Br zinc, Zn to form iron(II) ion, Fe . Thus, iron(III)
2
2+
is reduced to bromide ions, Br . ion, Fe is reduced to iron(II) ion, Fe .
3+
–
©PAN ASIA PUBLICATIONS
Oxidation half equation: Oxidation half equation:
Fe (aq) → Fe (aq) + e – Zn(s) → Zn (aq) + 2e –
2+
3+
2+
Reduction half equation: Reduction half equation:
Br (aq) + 2e → 2Br (aq) 3+ – 2+
–
–
2 Fe (aq) + e → Fe (aq)
Overall ionic equation (Redox reaction): Overall ionic equation (Redox reaction):
2+
–
3+
2Fe (aq) + Br (aq) → 2Fe (aq) + 2Br (aq) 3+ 2+ 2+
2 2Fe (aq) + Zn(s) → 2Fe (aq) + Zn (aq)
Try Question 7 in Formative Zone 1.1
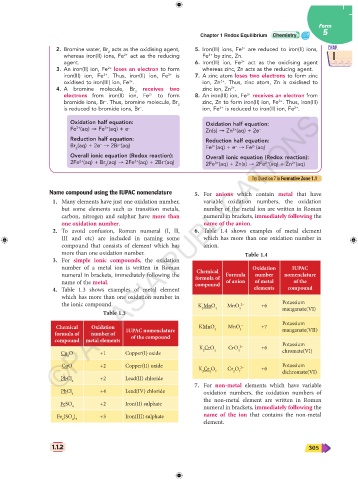
Name compound using the IUPAC nomenclature 5. For anions which contain metal that have
1. Many elements have just one oxidation number, variable oxidation numbers, the oxidation
but some elements such as transition metals, number of the metal ion are written in Roman
carbon, nitrogen and sulphur have more than numeral in brackets, immediately following the
one oxidation number. name of the anion.
2. To avoid confusion, Roman numeral (I, II, 6. Table 1.4 shows examples of metal element
III and etc) are included in naming some which has more than one oxidation number in
compound that consists of element which has anion.
more than one oxidation number. Table 1.4
3. For simple ionic compounds, the oxidation
number of a metal ion is written in Roman Chemical Oxidation IUPAC
numeral in brackets, immediately following the formula of Formula number nomenclature
name of the metal. compound of anion of metal of the
4. Table 1.3 shows examples of metal element elements compound
which has more than one oxidation number in
the ionic compound. K MnO 4 MnO 4 2– +6 Potassium
manganate(VI)
2
Table 1.3
Potassium
Chemical Oxidation IUPAC nomenclature KMnO 4 MnO 4 – +7 manganate(VII)
formula of number of of the compound
compound metal elements
K CrO CrO 2– +6 Potassium
Cu O +1 Copper(I) oxide 2 4 4 chromate(VI)
2
CuO +2 Copper(II) oxide Potassium
K Cr O 7 Cr O 7 2– +6 dichromate(VI)
2
2
2
PbCl 2 +2 Lead(II) chloride
7. For non-metal elements which have variable
PbCl +4 Lead(IV) chloride
4 oxidation numbers, the oxidation numbers of
the non-metal element are written in Roman
FeSO +2 Iron(II) sulphate
4 numeral in brackets, immediately following the
Fe (SO ) +3 Iron(III) sulphate name of the ion that contains the non-metal
2 4 3
element.
1.1.2 305