Page 26 - Spotlight A+ Form 4 & 5 Chemistry KSSM
P. 26
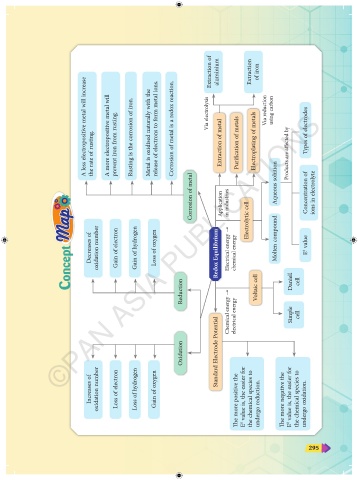
Extraction of aluminium Extraction of iron
A less electropositive metal will increase the rate of rusting. A more electropositive metal will prevent iron from rusting. Rusting is the corrosion of iron. Metal is oxidised naturally with the release of electrons to form metal ions. Corrosion of metal is a redox reaction. Via electrolysis Extraction of metal Purification of metals Electroplating of metals Via reduction using carbon Products are affected by Types
©PAN ASIA PUBLICATIONS
Corrosion of metal Application in industries Electrolytic cell Aqueous solution Concentration of ions in electrolyte
Decreases of oxidation number Gain of electron Gain of hydrogen Loss of oxygen Redox Equilibrium Electrical energy → chemical energy Molten compound E 0 value
Reduction Chemical energy → electrical energy Voltaic cell Daniell Simple cell cell
Oxidation Standard Electrode Potential
Increases of oxidation number Loss of electron Loss of hydrogen Gain of oxygen The more positive the E 0 value is, the easier for the chemical species to undergo reduction. The more negative the E 0 value is, the easier for the chemical species to undergo oxidation.
295