Page 906 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 906
Chapter 56 Conventional and Molecular Cytogenomic Basis of Hematologic Malignancies 789
“Preleukemic” Ph-negative stage Ph-positive stage, Acute (Blast)
chronic phase phase
A
B A
A B A A Myeloid
A A Erythroid +Ph,+8,+19,i (17q)
B Platelet BCR BCR
A A ABL ABL
Lymphoid
(T and B)
22
Stem cell Clonal Differentiation Genetic Fusion of t(9;22) Additional
development instability BCR/ABL karyotypic
abnormalities
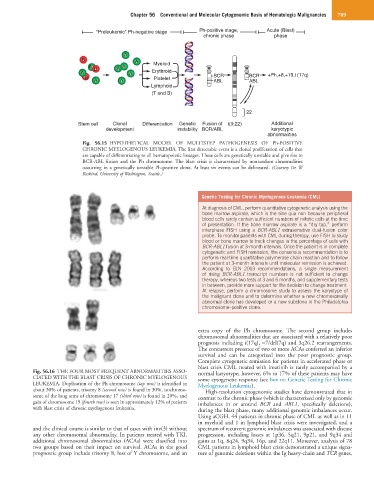
Fig. 56.15 HYPOTHETICAL MODEL OF MULTISTEP PATHOGENESIS OF Ph-POSITIVE
CHRONIC MYELOGENOUS LEUKEMIA. The first detectable event is a clonal proliferation of cells that
are capable of differentiating to all hematopoietic lineages. These cells are genetically unstable and give rise to
BCR-ABL fusion and the Ph chromosome. The blast crisis is characterized by nonrandom abnormalities
occurring in a genetically unstable Ph-positive clone. At least six events can be delineated. (Courtesy Dr. W
Raskind, University of Washington, Seattle.)
Genetic Testing for Chronic Myelogenous Leukemia (CML)
At diagnosis of CML, perform quantitative cytogenetic analysis using the
bone marrow aspirate, which is the sine qua non because peripheral
blood cells rarely contain sufficient numbers of mitotic cells at the time
of presentation. If the bone marrow aspirate is a “dry tap,” perform
interphase FISH using a BCR-ABL1 extrasensitive dual-fusion color
probe. To monitor patients with CML during therapy, use FISH to study
blood or bone marrow to track changes in the percentage of cells with
BCR-ABL1 fusion at 3-month intervals. Once the patient is in complete
cytogenetic and FISH remission, the consensus recommendation is to
perform real-time quantitative polymerase chain reaction and to follow
the patient at 3-month intervals until molecular remission is achieved.
According to ELN 2013 recommendations, a single measurement
of rising BCR-ABL1 transcript numbers is not sufficient to change
therapy, whereas two tests at 3 and 6 months, and supplementary tests
in between, provide more support for the decision to change treatment.
At relapse, perform a chromosome study to assess the karyotype of
the malignant clone and to determine whether a new chromosomally
abnormal clone has developed or a new subclone in the Philadelphia
chromosome–positive clone.
extra copy of the Ph chromosome. The second group includes
chromosomal abnormalities that are associated with a relatively poor
prognosis including i(17q), −7/del(7q) and 3q26.2 rearrangements.
The concurrent presence of two or more ACAs conferred an inferior
survival and can be categorized into the poor prognostic group.
Complete cytogenetic remission for patients in accelerated phase or
blast crisis CML treated with imatinib is rarely accompanied by a
Fig. 56.16 THE FOUR MOST FREQUENT ABNORMALITIES ASSO- normal karyotype; however, 6% to 17% of these patients may have
CIATED WITH THE BLAST CRISIS OF CHRONIC MYELOGENOUS some cytogenetic response (see box on Genetic Testing for Chronic
LEUKEMIA. Duplication of the Ph chromosome (top row) is identified in Myelogenous Leukemia).
about 30% of patients, trisomy 8 (second row) is found in 30%, isochromo- High-resolution cytogenomic studies have demonstrated that in
some of the long arms of chromosome 17 (third row) is found in 20%, and contrast to the chronic phase (which is characterized only by genomic
gain of chromosome 19 (fourth row) is seen in approximately 12% of patients imbalances in or around BCR and ABL1, specifically deletions),
with blast crisis of chronic myelogenous leukemia. during the blast phase, many additional genomic imbalances occur.
Using aCGH, 44 patients in chronic phase of CML as well as in 11
in myeloid and 1 in lymphoid blast crisis were investigated, and a
and the clinical course is similar to that of cases with inv(3) without spectrum of recurrent genomic imbalances was associated with disease
any other chromosomal abnormality. In patients treated with TKI, progression, including losses at 1p36, 5q21, 9p21, and 9q34 and
additional chromosomal abnormalities (ACAs) were classified into gains at 1q, 8q24, 9q34, 16p, and 22q11. Moreover, analysis of 78
two groups based on their impact on survival. ACAs in the good CML patients in lymphoid blast crisis demonstrated a unique signa-
prognostic group include trisomy 8, loss of Y chromosome, and an ture of genomic deletions within the Ig heavy-chain and TCR genes,