Page 911 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 911
794 Part VII Hematologic Malignancies
TABLE Recurrent Chromosomal Abnormalities in Primary
56.4 Myelodysplastic Syndrome
Abnormality Frequency (%)
−5 or del 5q 10–15
−7 or del 7q 10
trisomy 8 10–17
i(17q) or t(17p) 2–3
del(12p) or t(12p) 1–2
del(11q) 1–2
−13 or del(13q) 1–2
del(9q) 1–2
idic(X) 1
inv(3)(q21q26.2) 1
t(6;9)(p23;q34) 1
t(3;21)(q26.2;q22.1) <1
t(1;3)(p36.3;q21.2) <1
t(11;16)(q23;p13.3) <1
t(2;11) (p21;q23) <1
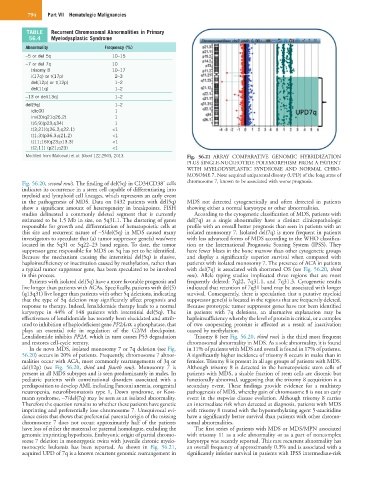
Modified from Malcovati et al: Blood 122:2943, 2013. Fig. 56.21 ARRAY COMPARATIVE GENOMIC HYBRIDIZATION
PLUS SINGLE-NUCLEOTIDE POLYMORPHISM FROM A PATIENT
WITH MYELODYSPLASTIC SYNDROME AND NORMAL CHRO-
MOSOME 7. Note acquired uniparental disomy (UPD) of the long arms of
−
Fig. 56.20, second row). The finding of del(5q) in CD34CD38 cells chromosome 7, known to be associated with worse prognosis.
indicates its occurrence in a stem cell capable of differentiating into
myeloid and lymphoid cell lineages, which represents an early event
in the pathogenesis of MDS. Data on 1432 patients with del(5q) MDS not detected cytogenetically and often detected in patients
show a significant amount of heterogeneity in breakpoints. FISH showing either a normal karyotype or other abnormalities.
studies delineated a commonly deleted segment that is currently According to the cytogenetic classification of MDS, patients with
estimated to be 1.5 Mb in size, on 5q31.1. The clustering of genes del(7q) as a single abnormality have a distinct clinicopathologic
responsible for growth and differentiation of hematopoietic cells at profile with an overall better prognosis than seen in patients with an
this site and recurrent nature of −5/del(5q) in MDS caused many isolated monosomy 7. Isolated del(7q) is more frequent in patients
investigators to speculate that (a) tumor suppressor gene(s) was/were with less advanced forms of MDS according to the WHO classifica-
located in the 5q31 or 5q22–23 band region. To date, the tumor tion or the International Prognostic Scoring System (IPSS). They
suppressor gene responsible for MDS on 5q has yet to be identified. have fewer blasts in the bone marrow than other cytogenetic groups
Because the mechanism causing the interstitial del(5q) is elusive, and display a significantly superior survival when compared with
haploinsufficiency or inactivation caused by methylation, rather than patients with isolated monosomy 7. The presence of ACA in patients
a typical tumor suppressor gene, has been speculated to be involved with del(7q) is associated with shortened OS (see Fig. 56.20, third
in this process. row). Allele typing studies implicated three regions that are most
Patients with isolated del(5q) have a more favorable prognosis and frequently deleted: 7q22, 7q31.1, and 7q31.3. Cytogenetic results
live longer than patients with ACAs. Specifically, patients with del(5) indicated that retention of 7q31 band may be associated with longer
(q13q31) live longer than patients with other 5q deletions, indicating survival. Consequently, there is speculation that a putative myeloid
that the type of 5q deletion may significantly affect prognosis and suppressor gene(s) is located in the regions that are frequently deleted.
response to therapy. Indeed, lenalidomide therapy leads to a normal Because prototypic tumor suppressor genes have not been identified
karyotype in 44% of 148 patients with interstitial del(5q). The in patients with 7q deletions, an alternative explanation may be
effectiveness of lenalidomide has recently been elucidated and attrib- haploinsufficiency whereby the level of protein is critical, or a complex
uted to inhibition of haplodeficient gene PP2Acα, a phosphatase, that of two cooperating proteins is affected as a result of inactivation
plays an essential role in regulation of the G2/M checkpoint. caused by methylation.
Lenalidomide inhibits PP2A, which in turn causes P53 degradation Trisomy 8 (see Fig. 56.20, third row) is the third most frequent
and restores cell-cycle reentry. chromosomal abnormality in MDS. As a sole abnormality, it is found
In de novo MDS, isolated monosomy 7 or 7q deletion (see Fig. in 11% of patients with MDS and overall is found in 17% of patients.
56.20) occurs in 20% of patients. Frequently, chromosome 7 abnor- A significantly higher incidence of trisomy 8 occurs in males than in
malities occur with ACA, most commonly rearrangements of 3q or females. Trisomy 8 is present in all age groups of patients with MDS.
del(12p) (see Fig. 56.20, third and fourth row). Monosomy 7 is Although trisomy 8 is detected in the hematopoietic stem cells of
present in all MDS subtypes and is seen predominantly in males. In patients with MDS, a sizable fraction of stem cells are disomic but
pediatric patients with constitutional disorders associated with a functionally abnormal, suggesting that the trisomy 8 acquisition is a
predisposition to develop AML including Fanconi anemia, congenital secondary event. These findings provide evidence for a multistep
neutropenia, neurofibromatosis type 1, Down syndrome, or Kost- pathogenesis of MDS, whereby gain of chromosome 8 is not an early
mann syndrome, −7/del(7q) may be seen as an isolated abnormality. event in the stepwise disease evolution. Although trisomy 8 carries
Therefore the question remains to whether these patients have genetic an intermediate risk when detected at diagnosis, patients with MDS
imprinting and preferentially lose chromosome 7. Unequivocal evi- with trisomy 8 treated with the hypomethylating agent 5-azacitidine
dence exists that shows that preferential parental origin of the missing have a significantly better survival than patients with other chromo-
chromosome 7 does not occur; approximately half of the patients somal abnormalities.
have loss of either the maternal or paternal homologue, excluding the The first series of patients with MDS or MDS/MPN associated
genomic imprinting hypothesis. Embryonic origin of partial chromo- with trisomy 11 as a sole abnormality or as a part of noncomplex
some 7 deletion in monozygotic twins with juvenile chronic myelo- karyotype was recently reported. This rare recurrent abnormality has
monocytic leukemia has been reported. As shown in Fig. 56.21, an overall frequency of approximately 0.3% and is associated with a
acquired UPD of 7q is a known recurrent genomic rearrangement in significantly inferior survival in patients with IPSS intermediate-risk