Page 916 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 916
Chapter 56 Conventional and Molecular Cytogenomic Basis of Hematologic Malignancies 799
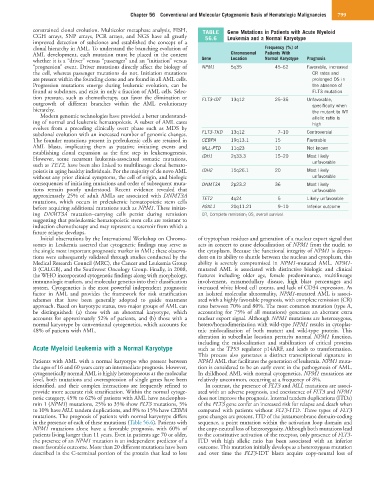
constrained clonal evolution. Multicolor metaphase analysis, FISH, TABLE Gene Mutations in Patients with Acute Myeloid
CGH arrays, SNP arrays, PCR arrays, and NGS have all greatly 56.6 Leukemia and a Normal Karyotype
improved detection of subclones and established the concept of a
clonal hierarchy in AML. To understand the branching evolution of Frequency (%) of
AML development, each mutation must be placed in the context Chromosomal Patients With
whether it is a “driver” versus “passenger” and an “initiation” versus Gene Location Normal Karyotype Prognosis
“progression” event. Driver mutations directly affect the biology of NPM1 5q35 45–62 Favorable, increased
the cell, whereas passenger mutations do not. Initiation mutations CR rates and
are present within the founding clone and are found in all AML cells. prolonged OS in
Progression mutations emerge during leukemic evolution, can be the absence of
found as subclones, and exist in only a fraction of AML cells. Selec- FLT3 mutation
tion pressure, such as chemotherapy, can favor the elimination or FLT3-IDT 13q12 25–35 Unfavorable,
outgrowth of different branches within the AML evolutionary specifically when
hierarchy. the mutant to WT
Modern genomic technologies have provided a better understand- allelic ratio is
ing of normal and leukemic hematopoiesis. A subset of AML cases high
evolves from a preceding clinically overt phase such as MDS by
subclonal evolution with an increased number of genomic changes. FLT3-TKD 13q12 7–10 Controversial
The founder mutations present in preleukemic cells are retained in CEBPA 19q13.1 15 Favorable
AML blasts, implicating them as putative initiating events and MLL-PTD 11q23 10 Not known
establishing clonal expansion as the first step in leukemogenesis.
However, some recurrent leukemia-associated somatic mutations, IDH1 2q33.3 15–20 Most likely
such as TET2, have been also linked to multilineage clonal hemato- unfavorable
poiesis in aging healthy individuals. For the majority of de novo AML IDH2 15q26.1 20 Most likely
without any prior clinical symptoms, the cell of origin, and biologic unfavorable
consequences of initiating mutations and order of subsequent muta- DNMT3A 2p23.2 36 Most likely
tions remain poorly understood. Recent evidence revealed that unfavorable
approximately 25% of adult AMLs are associated with DNMT3A TET2 4q24 5 Likely unfavorable
mutations, which occurs in preleukemic hematopoietic stem cells
before acquiring additional mutations such as NPM1. These initiat- ASXL1 20q11.21 9–10 Inferior outcome
ing DNMT3A mutation–carrying cells persist during remission CR, Complete remission; OS, overall survival.
suggesting that preleukemic hematopoietic stem cells are resistant to
induction chemotherapy and may represent a reservoir from which a
future relapse develops.
Initial observations by the International Workshop on Chromo- of tryptophan residues and generation of a nuclear export signal that
somes in Leukemia asserted that cytogenetic findings may serve as acts in concert to cause delocalization of NPM1 from the nuclei to
the single most important prognostic marker in AML; these observa- the cytoplasm. Because the functional integrity of NPM1 is depen-
tions were subsequently validated through studies conducted by the dent on its ability to shuttle between the nucleus and cytoplasm, this
Medical Research Council (MRC), the Cancer and Leukemia Group ability is severely compromised in NPM1-mutated AML. NPM1-
B (CALGB), and the Southwest Oncology Group. Finally, in 2008, mutated AML is associated with distinctive biologic and clinical
the WHO incorporated cytogenetic findings along with morphology, features including older age, female predominance, multilineage
immunologic markers, and molecular genetics into their classification involvement, extramedullary disease, high blast percentages and
system. Cytogenetics is the most powerful independent prognostic increased white blood cell counts, and lack of CD34 expression. As
factor in AML and provides the framework for risk stratification an isolated molecular abnormality, NPM1-mutated AML is associ-
schemes that have been generally adopted to guide treatment ated with a highly favorable prognosis, with complete remission (CR)
approach. Based on karyotype status, two major groups of AML can rates between 70% and 80%. The most common mutation (type A,
be distinguished: (a) those with an abnormal karyotype, which accounting for 75% of all mutations) generates an aberrant extra
accounts for approximately 52% of patients, and (b) those with a nuclear export signal. Although NPM1 mutations are heterozygous,
normal karyotype by conventional cytogenetics, which accounts for hetero/homodimerization with wild-type NPM1 results in cytoplas-
48% of patients with AML. mic mislocalization of both mutant and wild-type protein. This
alteration in subcellular location perturbs normal NPM1 function,
including the mislocalization and stabilization of critical proteins
Acute Myeloid Leukemia with a Normal Karyotype such as the TP53 regulator p14ARF, and leads to transformation.
This process also generates a distinct transcriptional signature in
Patients with AML with a normal karyotype who present between NPM1 AML that facilitates the generation of leukemia. NPM1 muta-
the ages of 16 and 60 years carry an intermediate prognosis. However, tion is considered to be an early event in the pathogenesis of AML.
cytogenetically normal AML is highly heterogeneous at the molecular In childhood AML with normal cytogenetics, NPM1 mutations are
level, both mutations and overexpression of single genes have been relatively uncommon, occurring at a frequency of 8%.
identified, and their complex interactions are frequently refined to In contrast, the presence of FLT3 and MLL mutations are associ-
provide more accurate risk stratification. Within the normal cytoge- ated with an adverse prognosis, and coexistence of FLT3 and NPM1
netic category, 45% to 62% of patients with AML have nucleophos- does not improve the prognosis. Internal tandem duplications (ITDs)
min 1 (NPM1) mutations, 25% to 35% show FLT3 mutations, 5% of the FLT3 gene confer an increased risk for relapse and death when
to 10% have MLL tandem duplications, and 8% to 15% have CEBPA compared with patients without FLT3-ITD. Three types of FLT3
mutations. The prognosis of patients with normal karyotype differs gene changes are present, ITD of the juxtamembrane domain-coding
in the presence of each of these mutations (Table 56.6). Patients with sequence, a point mutation within the activation loop domain and
NPM1 mutations alone have a favorable prognosis, with 60% of the copy-neutral loss of heterozygosity. Although both mutations lead
patients living longer than 11 years. Even in patients age 70 or older, to the constitutive activation of the receptor, only presence of FLT3-
the presence of an NPM1 mutation is an independent predictor of a ITD with high allelic ratio has been associated with an inferior
more favorable outcome. More than 20 different mutations have been outcome. This mutation initially develops as a heterozygous mutation
described in the C-terminal portion of the protein that lead to loss and over time the FLT3-IDT blasts acquire copy-neutral loss of