Page 1302 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 1302
CHAPTER 96: Sickle Cell Disease 909
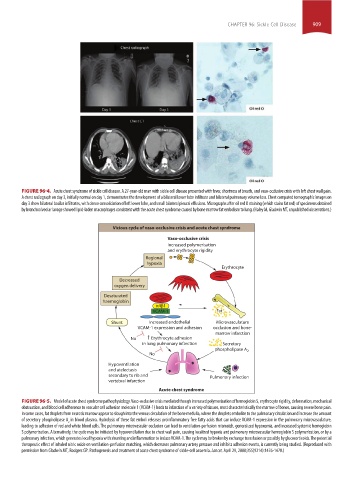
FIGURE 96-4. Acute chest syndrome of sickle cell disease. A 27-year-old man with sickle cell disease presented with fever, shortness of breath, and vaso-occlusive crisis with left chest wall pain.
A chest radiograph on day 3, initially normal on day 1, demonstrates the development of a bilateral lower lobe infiltrate and bilateral pulmonary volume loss. Chest computed tomographic images on
day 3 show bilateral basilar infiltrates, with dense consolidation of left lower lobe, and small bilateral pleural effusions. Micrographs after oil red O staining (which stains fat red) of specimens obtained
by bronchoalveolar lavage showed lipid-laden macrophages consistent with the acute chest syndrome caused by bone marrow fat embolism to lung. (Haley M, Gladwin MT, unpublished observations.)
Vicious cycle of vaso-occlusive crisis and acute chest syndrome
Vaso-occlusive crisis
Increased polymerisation
and erythrocyte rigidity
Regional
hypoxia
Erythrocyte
Decreased
oxygen delivery
Desaturated
haemoglobin
4 1
VCAM-1 Fat
Shunt Increased endothelial Microvasculature
VCAM-1 expression and adhesion occlusion and bone-
marrow infarction
No Erythrocyte adhesion
in lung pulmonary infarction Secretory
phospholipase A 2
No
Hypoventilation
and atelectasis
secondary to rib and Pulmonary infection
vertebral infarction
Acute chest syndrome
FIGURE 96-5. Model of acute chest syndrome pathophysiology. Vaso-occlusive crisis mediated though increased polymerization of hemoglobin S, erythrocyte rigidity, deformation, mechanical
obstruction, and blood cell adherence to vascular cell adhesion molecule 1 (VCAM-1) leads to infarction of a variety of tissues, most characteristically the marrow of bones, causing severe bone pain.
In some cases, fat droplets from necrotic marrow appear to slough into the venous circulation of the bone medulla, where the droplets embolize to the pulmonary circulation and increase the amount
of secretory phospholipase A in blood plasma. Hydrolysis of these fat emboli releases proinflammatory free fatty acids that can induce VCAM-1 expression in the pulmonary microvasculature,
2
leading to adhesion of red and white blood cells. The pulmonary microvascular occlusion can lead to ventilation-perfusion mismatch, generalized hypoxemia, and increased systemic hemoglobin
S polymerization. Alternatively, the cycle may be initiated by hypoventilation due to chest wall pain, causing localized hypoxia and pulmonary microvascular hemoglobin S polymerization, or by a
pulmonary infection, which generates local hypoxia with shunting and inflammation to induce VCAM-1. The cycle may be broken by exchange transfusion or possibly by glucocorticoids. The potential
therapeutic effect of inhaled nitric oxide on ventilation-perfusion matching, which decreases pulmonary artery pressure and inhibits adhesion events, is currently being studied. (Reproduced with
permission from Gladwin MT, Rodgers GP. Pathogenesis and treatment of acute chest syndrome of sickle-cell anaemia. Lancet. April 29, 2000;355(9214):1476-1478.)
section07.indd 909 1/21/2015 7:43:24 AM