Page 650 - Clinical Hematology_ Theory _ Procedures ( PDFDrive )
P. 650
634 PART 8 ■ Fundamentals of Hematological Analysis
as a stain, using a similar method to that o the peroxidase General Properties of Flow Cytometry
channel. Lymphocytes, which have been labeled by the
immunoperoxidase reaction, appear between the unla- Most f ow-cell instruments can simultaneously analyze mul-
beled lymphocyte population and the monocytes. Cells with tiple parameters at the rate o 5,000 to 10,000 cells/second.
endogenous peroxidase such as neutrophils stain intensely T e cellular analysis yields quantitative data about the chem-
and appear ar to the right. ical and physical properties o individual cells, and a er anal-
ysis, cells can be physically separated into subpopulations
or urther study at the rate o 5,000 cells/second. T e major
APPLICATIONS OF FLOW CYTOMETRY advances in this technology are owing to several actors:
T e introduction o the f ow cytometer into the clinical labo- 1. T e ability to produce monoclonal antibodies resulted in
ratory is a major technological advance. Flow cytometry is a the subsequent development o speci c sur ace markers
eld that has evolved rapidly during the past three decades. or various subpopulations o cells.
Instruments based on the f ow cytometry principle were ini- 2. T e development o new f uorescent probes or DNA,
tially designed to count and size cells. Later modi cations RNA, and other cellular components increased the variety
were designed to per orm di erential leukocyte counts by o possible applications at the molecular and cellular level.
identi ying speci c cytochemical reactions in the cells. T e 3. T e expansion o computer applications has improved the
current types o f ow cytometry instruments can analyze instrumentation technology, making it easier to operate
cells or many constituents and sort cells into subpopulations and more practical or use in clinical as well as research
(Fig. 30.20). laboratories.
Hematological Applications
Flow cytometry can be applied practically to several tech-
niques in the clinical hematology laboratory. T ese appli-
cations include automated leukocyte di erentiation and
reticulocyte enumeration.
Autom ated Differentials
Automated di erentials can be based on a variety o princi-
ples. T ese include determination o cell volume by electrical
impedance or orward light scatter, cytochemistry or per-
oxidase staining, and VCS technology. Evaluation o internal
cellular organelles and nuclear characteristics can be by:
■ 90-Degree laser scatter
■ Polarizing laser light
■ RF
Separate measurements can be made o individual mea-
surements o volume, conductivity, and light scatter. An
additional method is to integrate the three in VCS technol-
ogy into a three-dimensional (3D) leukocyte analysis. T e
volume aspect is by volumetric sizing by impedance and RF
opacity or internal composition. In addition, helium-neon
laser light scatter is applied so that laser light can produce
scattering characteristics o each cell at di erent angles or
granularity and nuclear structure.
In addition, di erent reagents can be used to lyse certain
cells. Di erent types o technologies are used by instrument
manu acturers to produce an automated leukocyte di eren-
tial. T ese include the ollowing:
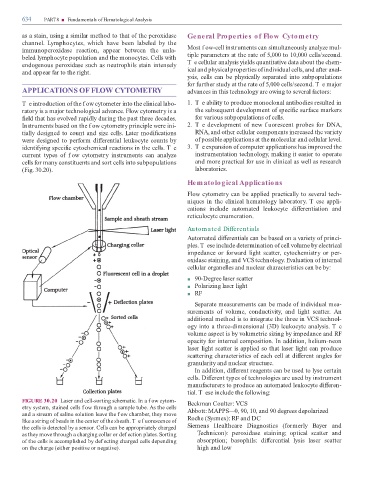
FIGURE 30.20 Laser and cell-sorting schematic. In a f ow cytom- Beckman Coulter: VCS
etry system, stained cells f ow through a sample tube. As the cells
and a stream o saline solution leave the f ow chamber, they move Abbott: MAPPS—0, 90, 10, and 90 degrees depolarized
like a string o beads in the center o the sheath. T e f uorescence o Roche (Sysmex): RF and DC
the cells is detected by a sensor. Cells can be appropriately charged Siemens Healthcare Diagnostics ( ormerly Bayer and
as they move through a charging collar or def ection plates. Sorting echnicon): peroxidase staining; optical scatter and
o the cells is accomplished by def ecting charged cells depending absorption; basophils: di erential lysis laser scatter
on the charge (either positive or negative). high and low